Budding yeast CENP-ACse4 interacts with the N-terminus of Sgo1 and regulates its association with centromeric chromatin
- PMID: 28980861
- PMCID: PMC5815428
- DOI: 10.1080/15384101.2017.1380129
Budding yeast CENP-ACse4 interacts with the N-terminus of Sgo1 and regulates its association with centromeric chromatin
Abstract
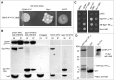
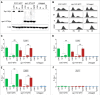
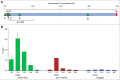
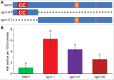
Shugoshin is an evolutionarily conserved protein, which is involved in tension sensing on mitotic chromosomes, kinetochore biorientation, and protection of centromeric (CEN) cohesin for faithful chromosome segregation. Interaction of the C-terminus of Sgo1 with phosphorylated histone H2A regulates its association with CEN and pericentromeric (peri-CEN) chromatin, whereas mutations in histone H3 selectively compromise the association of Sgo1 with peri-CEN but not CEN chromatin. Given that histone H3 is absent from CEN and is replaced by a histone H3 variant CENP-ACse4, we investigated if CENP-ACse4 interacts with Sgo1 and promotes its association with the CEN chromatin. In this study, we found that Sgo1 interacts with CENP-ACse4 in vivo and in vitro. The N-terminus coiled-coil domain of Sgo1 without the C-terminus (sgo1-NT) is sufficient for its interaction with CENP-ACse4, association with CEN but not the peri-CEN, and this CEN association is cell cycle dependent with maximum enrichment in mitosis. In agreement with the role of CENP-ACse4 in CEN maintenance of Sgo1, depletion of CENP-ACse4 results in the loss of Sgo1 and sgo1-NT from the CEN chromatin. The N-terminus of Sgo1 is required for genome stability as a mutant lacking the N-terminus (sgo1-CT) exhibits increased chromosome missegregation when compared to a sgo1-NT mutant. In summary, our results define a novel role for the N-terminus of Sgo1 in CENP-ACse4 mediated recruitment of Sgo1 to CEN chromatin for faithful chromosome segregation.
Keywords: CENP-A; Cse4; Sgo1; Shugoshin; cell cycle; centromere; gene regulation; kinetochore; mitosis; yeast.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
