Calcium/calmodulin-dependent kinase kinase 2 regulates hematopoietic stem and progenitor cell regeneration
- PMID: 28981105
- PMCID: PMC5680595
- DOI: 10.1038/cddis.2017.474
Calcium/calmodulin-dependent kinase kinase 2 regulates hematopoietic stem and progenitor cell regeneration
Abstract
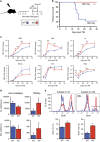
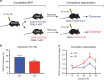
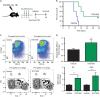
Hematopoietic stem and progenitor cells (HSPCs) are predominantly quiescent in adults, but proliferate in response to bone marrow (BM) injury. Here, we show that deletion of Ca2+/calmodulin (CaM)-dependent protein kinase kinase 2 (CaMKK2) promotes HSPC regeneration and hematopoietic recovery following radiation injury. Using Camkk2-enhanced green fluorescent protein (EGFP) reporter mice, we found that Camkk2 expression is developmentally regulated in HSPC. Deletion of Camkk2 in HSPC results in a significant downregulation of genes affiliated with the quiescent signature. Accordingly, HSPC from Camkk2 null mice have a high proliferative capability when stimulated in vitro in the presence of BM-derived endothelial cells. In addition, Camkk2 null mice are more resistant to radiation injury and show accelerated hematopoietic recovery, enhanced HSPC regeneration and ultimately a prolonged survival following sublethal or lethal total body irradiation. Mechanistically, we propose that CaMKK2 regulates the HSPC response to hematopoietic damage by coupling radiation signaling to activation of the anti-proliferative AMP-activated protein kinase. Finally, we demonstrated that systemic administration of the small molecule CaMKK2 inhibitor, STO-609, to irradiated mice enhanced HSPC recovery and improved survival. These findings identify CaMKK2 as an important regulator of HSPC regeneration and demonstrate CaMKK2 inhibition is a novel approach to promoting hematopoietic recovery after BM injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425: 836–841. - PubMed
-
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 2010; 116: 4815–4828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

