Cofilin1-dependent actin dynamics control DRP1-mediated mitochondrial fission
- PMID: 28981113
- PMCID: PMC5680571
- DOI: 10.1038/cddis.2017.448
Cofilin1-dependent actin dynamics control DRP1-mediated mitochondrial fission
Abstract
Mitochondria form highly dynamic networks in which organelles constantly fuse and divide. The relevance of mitochondrial dynamics is evident from its implication in various human pathologies, including cancer or neurodegenerative, endocrine and cardiovascular diseases. Dynamin-related protein 1 (DRP1) is a key regulator of mitochondrial fission that oligomerizes at the mitochondrial outer membrane and hydrolyzes GTP to drive mitochondrial fragmentation. Previous studies demonstrated that DRP1 recruitment and mitochondrial fission is promoted by actin polymerization at the mitochondrial surface, controlled by the actin regulatory proteins inverted formin 2 (INF2) and Spire1C. These studies suggested the requirement of additional actin regulatory activities to control DRP1-mediated mitochondrial fission. Here we show that the actin-depolymerizing protein cofilin1, but not its close homolog actin-depolymerizing factor (ADF), is required to maintain mitochondrial morphology. Deletion of cofilin1 caused mitochondrial DRP1 accumulation and fragmentation, without altering mitochondrial function or other organelles' morphology. Mitochondrial morphology in cofilin1-deficient cells was restored upon (i) re-expression of wild-type cofilin1 or a constitutively active mutant, but not of an actin-binding-deficient mutant, (ii) pharmacological destabilization of actin filaments and (iii) genetic depletion of DRP1. Our work unraveled a novel function for cofilin1-dependent actin dynamics in mitochondrial fission, and identified cofilin1 as a negative regulator of mitochondrial DRP1 activity. We conclude that cofilin1 is required for local actin dynamics at mitochondria, where it may balance INF2/Spire1C-induced actin polymerization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

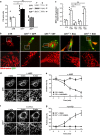
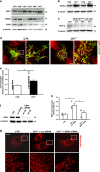
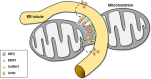
References
-
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 2003; 4: 552–565. - PubMed
-
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell 2004; 116: 205–219. - PubMed
-
- Bereiter-Hahn J, Vöth M, Mai S, Jendrach M. Structural implications of mitochondrial dynamics. Biotechnol J 2008; 3: 765–780. - PubMed
-
- Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 2010; 11: 872–884. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

