Challenges in Patient Enrollment and Retention in Clinical Studies for Alcoholic Hepatitis: Experience of the TREAT Consortium
- PMID: 28981151
- PMCID: PMC5711577
- DOI: 10.1111/acer.13515
Challenges in Patient Enrollment and Retention in Clinical Studies for Alcoholic Hepatitis: Experience of the TREAT Consortium
Abstract
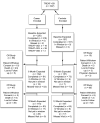
The TREAT Consortium has carried out clinical studies on alcoholic hepatitis (AH) for over 4 years. We encountered problems with participant recruitment, retention, and eligibility for specific protocols. To improve our ability to carry out such trials, we reviewed recruitment screening logs, end of study logs, and surveyed study coordinators to learn the reasons for missing patients, why patients declined enrollment, and the number of patients eligible for treatment trials. Associations of the recruited subjects' demographics with their adherence to follow-up appointments were examined. Three hundred eight-seven patients (AH and heavy drinking controls) were enrolled in the observational study, and 55 AH patients were recruited into treatment trials. About half of patients identified with AH could not be recruited; no specific reason could be determined for about two-thirds of these. Among the patients who gave a reason for not participating, the most common reasons were feeling too sick to participate, desire to concentrate on abstinence, and lack of interest in research. Approximately a quarter of the AH patients met eligibility criteria for treatment trials for moderate or severe AH and we were able to recruit half to two-thirds of those eligible. Approximately 35% of participants in the observational study returned for both 6- and 12-month follow-up visits. We did not identify biopsychosocial or demographic correlates of retention in the study. This analysis revealed that attempts at recruitment into trials for AH miss some subjects because of structural issues surrounding their hospital admission, and encounter a high rate of patient refusal to participate. Nonetheless, more than half of the patients who met the eligibility criteria for moderate or severe AH were entered into clinical trials. Retention rates for the observational study are relatively low. These findings need to be accounted for in clinical trial design and power analysis.
Keywords: Alcoholic Hepatitis; Clinical Trial; Model for End-Stage Liver Disease Score; Recruitment; Retention.
Copyright © 2017 by the Research Society on Alcoholism.
Conflict of interest statement
Figures
References
-
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D'Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–1922. - PubMed
-
- Buch S, Stickel F, Trepo E, Way M, Herrmann A, Nischalke HD, Brosch M, Rosendahl J, Berg T, Ridinger M, Rietschel M, McQuillin A, Frank J, Kiefer F, Schreiber S, Lieb W, Soyka M, Semmo N, Aigner E, Datz C, Schmelz R, Brückner S, Zeissig S, Stephan AM, Wodarz N, Devière J, Clumeck N, Sarrazin C, Lammert F, Gustot T, Deltenre P, Völzke H, Lerch MM, Mayerle J, Eyer F, Schafmayer C, Cichon S, Nöthen MM, Nothnagel M, Ellinghaus D, Huse K, Franke A, Zopf S, Hellerbrand C, Moreno C, Franchimont D, Morgan MY, Hampe J. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47:1443–1448. - PubMed
-
- Burza MA, Molinaro A, Attilia ML, Rotondo C, Attilia F, Ceccanti M, Ferri F, Maldarelli F, Maffongelli A, De SA, Attili AF, Romeo S, Ginanni CS. PNPLA3 I148M (rs738409) genetic variant and age at onset of at-risk alcohol consumption are independent risk factors for alcoholic cirrhosis. Liver Int. 2014;34:514–520. - PMC - PubMed
-
- Chamorro AJ, Torres JL, Miron-Canelo JA, Gonzalez-Sarmiento R, Laso FJ, Marcos M. Systematic review with meta-analysis: the I148M variant of patatin-like phospholipase domain-containing 3 gene (PNPLA3) is significantly associated with alcoholic liver cirrhosis. Aliment Pharmacol Ther. 2014;40:571–581. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

