Extracellular Cyclophilin A Augments Platelet-Dependent Thrombosis and Thromboinflammation
- PMID: 28981554
- PMCID: PMC5885247
- DOI: 10.1160/TH17-01-0067
Extracellular Cyclophilin A Augments Platelet-Dependent Thrombosis and Thromboinflammation
Abstract
Cyclophilin A (CyPA) is involved in the pathophysiology of several inflammatory and cardiovascular diseases. To our knowledge, there is no specific inhibitor targeting extracellular CyPA without affecting other extracellular cyclophilins or intracellular CyPA functions. In this study, we developed an antibody-based inhibitor of extracellular CyPA and analysed its effects in vitro and in vivo. To generate a specific antibody, mice and rats were immunized with a peptide containing the extracellular matrix metalloproteinase inducer binding site and various antibody clones were selected and purified. At first, antibodies were tested for their binding capacity to recombinant CyPA and their functional activity. The clone 8H7-mAb was chosen for further experiments. 8H7-mAb reduced the CyPA-induced migration of inflammatory cells in vitro and in vivo. Furthermore, 8H7-mAb revealed strong antithrombotic effects by inhibiting CyPA-dependent activation of platelets and thrombus formation in vitro and in vivo. Surprisingly, 8H7-mAb did not influence in vivo tail bleeding time or in vitro whole blood coagulation parameters. Our study provides first evidence that antibody-based inhibition of extracellular CyPA inhibits thrombosis and thromboinflammation without affecting blood homeostasis. Thus, 8H7-mAb may be a promising compound for thrombi modulation in inflammatory diseases to prevent organ dysfunction.
Schattauer GmbH Stuttgart.
Conflict of interest statement
Disclosure: None.
Figures
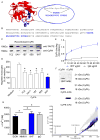
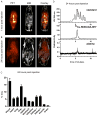
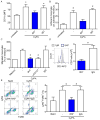
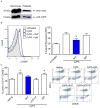


References
-
- Fischer G, Wittmann-Liebold B, Lang K, et al. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989;337(6206):476–8. - PubMed
-
- Galigniana MD, Morishima Y, Gallay PA, et al. Cyclophilin-A is bound through its peptidylprolyl isomerase domain to the cytoplasmic dynein motor protein complex. The Journal of biological chemistry. 2004;279(53):55754–9. - PubMed
-
- Rosado JA, Pariente JA, Salido GM, et al. SERCA2b activity is regulated by cyclophilins in human platelets. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(3):419–25. - PubMed
-
- Kern G, Kern D, Schmid FX, et al. Reassessment of the putative chaperone function of prolyl-cis/trans-isomerases. FEBS letters. 1994;348(2):145–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

