Neuron-Targeted Caveolin-1 Promotes Ultrastructural and Functional Hippocampal Synaptic Plasticity
- PMID: 28981594
- PMCID: PMC6095215
- DOI: 10.1093/cercor/bhx196
Neuron-Targeted Caveolin-1 Promotes Ultrastructural and Functional Hippocampal Synaptic Plasticity
Abstract
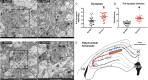
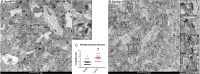
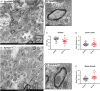
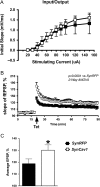
A delicate interneuronal communication between pre- and postsynaptic membranes is critical for synaptic plasticity and the formation of memory. Evidence shows that membrane/lipid rafts (MLRs), plasma membrane microdomains enriched in cholesterol and sphingolipids, organize presynaptic proteins and postsynaptic receptors necessary for synaptic formation and signaling. MLRs establish a cell polarity that facilitates transduction of extracellular cues to the intracellular environment. Here we show that neuron-targeted overexpression of an MLR protein, caveolin-1 (SynCav1), in the adult mouse hippocampus increased the number of presynaptic vesicles per bouton, total excitatory type I glutamatergic synapses, number of same-dendrite multiple-synapse boutons, increased myelination, increased long-term potentiation, and increased MLR-localized N-methyl-d-aspartate receptor subunits (GluN1, GluN2A, and GluN2B). Immunogold electron microscopy revealed that Cav-1 localizes to both the pre- and postsynaptic membrane regions as well as in the synaptic cleft. These findings, which are consistent with a significant increase in ultrastructural and functional synaptic plasticity, provide a fundamental framework that underlies previously demonstrated improvements in learning and memory in adult and aged mice by SynCav1. Such observations suggest that Cav-1 and MLRs alter basic aspects of synapse biology that could serve as potential therapeutic targets to promote neuroplasticity and combat neurodegeneration in a number of neurological disorders.
Figures


References
-
- Andersen P. 1960. Interhippocampal impulses. II. Apical dendritic activation of CAI neurons. Acta Physiol Scand. 48:178–208. - PubMed
-
- Andersen P, Blackstad TW, Lomo T. 1966. Location and identification of excitatory synapses on hippocampal pyramidal cells. Exp Brain Res. 1:236–248. - PubMed
-
- Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J. 2007. The Hippocampus Book. Oxford Neuroscience Series. New York:Oxford University Press.
-
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. 2005. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 8:1148–1150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

