Transcription Factors Sp8 and Sp9 Coordinately Regulate Olfactory Bulb Interneuron Development
- PMID: 28981617
- PMCID: PMC6095218
- DOI: 10.1093/cercor/bhx199
Transcription Factors Sp8 and Sp9 Coordinately Regulate Olfactory Bulb Interneuron Development
Abstract
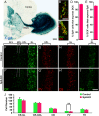
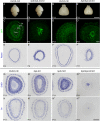
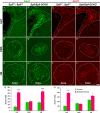
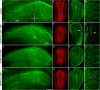
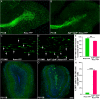
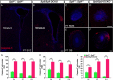
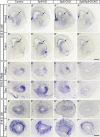
Neural stem cells in the postnatal telencephalic ventricular-subventricular zone (V-SVZ) generate new interneurons, which migrate tangentially through the rostral migratory stream (RMS) into the olfactory bulb (OB). The Sp8 and Sp9 transcription factors are expressed in neuroblasts, as well as in the immature and mature interneurons in the V-SVZ-RMS-OB system. Here we show that Sp8 and Sp9 coordinately regulate OB interneuron development: although Sp9 null mutants show no major OB interneuron defect, conditional deletion of both Sp8 and Sp9 resulted in a much more severe reduction of OB interneuron number than that observed in the Sp8 conditional mutant mice, due to defects in neuronal differentiation, tangential and radial migration, and increased cell death in the V-SVZ-RMS-OB system. RNA-Seq and RNA in situ hybridization reveal that, in Sp8/Sp9 double mutant mice, but not in Sp8 or Sp9 single mutant mice, newly born neuroblasts in the V-SVZ-RMS-OB system fail to express Prokr2 and Tshz1 expression, genes with known roles in promoting OB interneuron differentiation and migration, and that are involved in human Kallmann syndrome.
Figures

Similar articles
-
Temporally Distinct Roles for the Zinc Finger Transcription Factor Sp8 in the Generation and Migration of Dorsal Lateral Ganglionic Eminence (dLGE)-Derived Neuronal Subtypes in the Mouse.Cereb Cortex. 2021 Feb 5;31(3):1744-1762. doi: 10.1093/cercor/bhaa323. Cereb Cortex. 2021. PMID: 33230547 Free PMC article.
-
The PROK2/PROKR2 signaling pathway is required for the migration of most olfactory bulb interneurons.J Comp Neurol. 2019 Dec 15;527(18):2931-2947. doi: 10.1002/cne.24719. Epub 2019 Jun 13. J Comp Neurol. 2019. PMID: 31132148
-
Dlx1/2 are Central and Essential Components in the Transcriptional Code for Generating Olfactory Bulb Interneurons.Cereb Cortex. 2019 Dec 17;29(11):4831-4849. doi: 10.1093/cercor/bhz018. Cereb Cortex. 2019. PMID: 30796806 Free PMC article.
-
The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis.Cold Spring Harb Perspect Biol. 2016 May 2;8(5):a018820. doi: 10.1101/cshperspect.a018820. Cold Spring Harb Perspect Biol. 2016. PMID: 27048191 Free PMC article. Review.
-
Olfactory bulb neurogenesis depending on signaling in the subventricular zone.Cereb Cortex. 2023 Nov 4;33(22):11102-11111. doi: 10.1093/cercor/bhad349. Cereb Cortex. 2023. PMID: 37746807 Review.
Cited by
-
Tracking cell-type-specific temporal dynamics in human and mouse brains.Cell. 2023 Sep 28;186(20):4345-4364.e24. doi: 10.1016/j.cell.2023.08.042. Cell. 2023. PMID: 37774676 Free PMC article.
-
Decoding Cortical Glial Cell Development.Neurosci Bull. 2021 Apr;37(4):440-460. doi: 10.1007/s12264-021-00640-9. Epub 2021 Feb 19. Neurosci Bull. 2021. PMID: 33606177 Free PMC article.
-
Arx revisited: involved in the development of GABAergic interneurons.Front Cell Dev Biol. 2025 Mar 28;13:1563515. doi: 10.3389/fcell.2025.1563515. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40226590 Free PMC article. Review.
-
Temporally Distinct Roles for the Zinc Finger Transcription Factor Sp8 in the Generation and Migration of Dorsal Lateral Ganglionic Eminence (dLGE)-Derived Neuronal Subtypes in the Mouse.Cereb Cortex. 2021 Feb 5;31(3):1744-1762. doi: 10.1093/cercor/bhaa323. Cereb Cortex. 2021. PMID: 33230547 Free PMC article.
-
Neuroblasts contribute to oligodendrocytes generation upon demyelination in the adult mouse brain.iScience. 2022 Sep 13;25(10):105102. doi: 10.1016/j.isci.2022.105102. eCollection 2022 Oct 21. iScience. 2022. PMID: 36185360 Free PMC article.
References
-
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. 1997. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 19:27–37. - PubMed
-
- Bartolini G, Ciceri G, Marin O. 2013. Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron. 79:849–864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

