Embraced by eIF3: structural and functional insights into the roles of eIF3 across the translation cycle
- PMID: 28981723
- PMCID: PMC5737393
- DOI: 10.1093/nar/gkx805
Embraced by eIF3: structural and functional insights into the roles of eIF3 across the translation cycle
Abstract
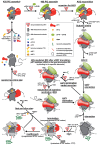
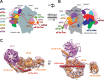
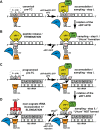
Protein synthesis is mediated via numerous molecules including the ribosome, mRNA, tRNAs, as well as translation initiation, elongation and release factors. Some of these factors play several roles throughout the entire process to ensure proper assembly of the preinitiation complex on the right mRNA, accurate selection of the initiation codon, errorless production of the encoded polypeptide and its proper termination. Perhaps, the most intriguing of these multitasking factors is the eukaryotic initiation factor eIF3. Recent evidence strongly suggests that this factor, which coordinates the progress of most of the initiation steps, does not come off the initiation complex upon subunit joining, but instead it remains bound to 80S ribosomes and gradually falls off during the first few elongation cycles to: (1) promote resumption of scanning on the same mRNA molecule for reinitiation downstream-in case of translation of upstream ORFs short enough to preserve eIF3 bound; or (2) come back during termination on long ORFs to fine tune its fidelity or, if signaled, promote programmed stop codon readthrough. Here, we unite recent structural views of the eIF3-40S complex and discus all known eIF3 roles to provide a broad picture of the eIF3's impact on translational control in eukaryotic cells.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

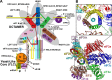

Similar articles
-
The RNA recognition motif of eukaryotic translation initiation factor 3g (eIF3g) is required for resumption of scanning of posttermination ribosomes for reinitiation on GCN4 and together with eIF3i stimulates linear scanning.Mol Cell Biol. 2010 Oct;30(19):4671-86. doi: 10.1128/MCB.00430-10. Epub 2010 Aug 2. Mol Cell Biol. 2010. PMID: 20679478 Free PMC article.
-
Eukaryotic translation initiation factor 3 (eIF3) and eIF2 can promote mRNA binding to 40S subunits independently of eIF4G in yeast.Mol Cell Biol. 2006 Feb;26(4):1355-72. doi: 10.1128/MCB.26.4.1355-1372.2006. Mol Cell Biol. 2006. PMID: 16449648 Free PMC article.
-
eIF3 Peripheral Subunits Rearrangement after mRNA Binding and Start-Codon Recognition.Mol Cell. 2016 Jul 21;63(2):206-217. doi: 10.1016/j.molcel.2016.05.033. Epub 2016 Jun 30. Mol Cell. 2016. PMID: 27373335
-
Does eIF3 promote reinitiation after translation of short upstream ORFs also in mammalian cells?RNA Biol. 2017 Dec 2;14(12):1660-1667. doi: 10.1080/15476286.2017.1353863. Epub 2017 Sep 15. RNA Biol. 2017. PMID: 28745933 Free PMC article. Review.
-
eIF3: a versatile scaffold for translation initiation complexes.Trends Biochem Sci. 2006 Oct;31(10):553-62. doi: 10.1016/j.tibs.2006.08.005. Epub 2006 Aug 22. Trends Biochem Sci. 2006. PMID: 16920360 Review.
Cited by
-
Stress-Induced Translation Inhibition through Rapid Displacement of Scanning Initiation Factors.Mol Cell. 2020 Nov 5;80(3):470-484.e8. doi: 10.1016/j.molcel.2020.09.021. Epub 2020 Oct 13. Mol Cell. 2020. PMID: 33053322 Free PMC article.
-
Eukaryotic translation initiation factor 3 subunit B could serve as a potential prognostic predictor for breast cancer.Bioengineered. 2022 Feb;13(2):2762-2776. doi: 10.1080/21655979.2021.2017567. Bioengineered. 2022. PMID: 35040374 Free PMC article.
-
uORF-mediated translational control: recently elucidated mechanisms and implications in cancer.RNA Biol. 2019 Oct;16(10):1327-1338. doi: 10.1080/15476286.2019.1632634. Epub 2019 Jun 24. RNA Biol. 2019. PMID: 31234713 Free PMC article. Review.
-
An interaction between eIF4A3 and eIF3g drives the internal initiation of translation.Nucleic Acids Res. 2023 Nov 10;51(20):10950-10969. doi: 10.1093/nar/gkad763. Nucleic Acids Res. 2023. PMID: 37811880 Free PMC article.
-
Uukuniemi virus infection causes a pervasive remodelling of the RNA-binding proteome in tick cells.PLoS Pathog. 2025 Aug 4;21(8):e1013393. doi: 10.1371/journal.ppat.1013393. eCollection 2025 Aug. PLoS Pathog. 2025. PMID: 40758761 Free PMC article.
References
-
- Hinnebusch A.G. Structural insights into the mechanism of scanning and start codon recognition in eukaryotic translation initiation. Trends Biochem. Sci. 2017; 42:589–611. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

