Novel CH1:CL interfaces that enhance correct light chain pairing in heterodimeric bispecific antibodies
- PMID: 28981885
- PMCID: PMC5914326
- DOI: 10.1093/protein/gzx044
Novel CH1:CL interfaces that enhance correct light chain pairing in heterodimeric bispecific antibodies
Abstract
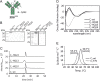
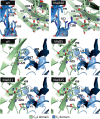
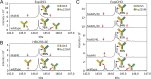
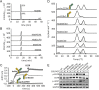
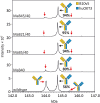
Targeting two unique antigens with a single bispecific antibody is an attractive approach with potential broad therapeutic applicability. However, the production of heterodimeric bispecific antibodies (bsAbs) presents a challenge, requiring the co-expression and accurate pairing of two distinct heavy and light chain units. Several undesirable by-products can be formed in the production process, including heavy chain homodimers and non-cognate light chain pairings. Although additional downstream purification methods exist, they are often time consuming and restrict practical large-scale production. In this study, we identify and validate novel Fab interface mutations that increase cognate light chain pairing efficiencies within heterodimeric bsAbs. Importantly, the variable domains remain unaltered as interface mutations were restricted to the CH1 and CL domains. We performed several biochemical assays to demonstrate that the novel engineered interfaces do not adversely impact bispecific antibody expression, stability, affinity and biological function. The designs reported here can easily be applied in a generic manner to use existing antibodies as building blocks for bsAbs which will help to accelerate the identification and production of next generation bispecific antibody therapeutics.
Keywords: Fab interface design; bispecific antibodies; heterodimeric IgG; light chain pairing problem; orthogonal Fab engineering.
© The Author 2017. Published by Oxford University Press.
Figures


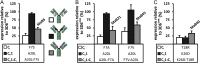
References
-
- Arnold K., Bordoli L., Kopp J. and Schwede T. (2006) Bioinforma. Oxf. Engl., 22, 195–201. - PubMed
-
- Atwell S., Ridgway J.B.B., Wells J.A. and Carter P. (1997) J. Mol. Biol., 270, 26–35. - PubMed
-
- Brezinschek H.P., Foster S.J., Dörner T., Brezinschek R.I. and Lipsky P.E. (1998) J. Immunol. Baltim. Md 1950, 160, 4762–4767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

