A Randomized Trial of Text Messaging for Smoking Cessation in Pregnant Women
- PMID: 28982527
- PMCID: PMC5696101
- DOI: 10.1016/j.amepre.2017.08.002
A Randomized Trial of Text Messaging for Smoking Cessation in Pregnant Women
Abstract
Introduction: There is a need for innovation in both the enrollment of pregnant smokers in smoking cessation treatment programs and in the types of treatments offered. The study tests whether an interactive and intensive text messaging program, Quit4baby, can promote smoking cessation for pregnant women already enrolled in a health text messaging program, Text4baby.
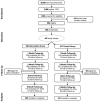
Methods: Between July 2015 and February 2016, a total of 35,957 recruitment text messages were sent to Text4baby subscribers. Eligible pregnant smokers were enrolled and randomized to receive Text4baby (control) or Text4baby and Quit4baby (intervention; N=497). Participants were surveyed at 1 month, 3 months, and 6 months post-enrollment, and saliva samples were collected at 3 months for biochemical verification of smoking status. Data were collected from 2015 to 2016 and analyzed in 2016.
Results: Using an intention-to-treat analysis, 28.80% of the intervention group and 15.79% of the control group reported not smoking in the past 7 days at 1 month (p<0.01), and 35.20% of the intervention group and 22.67% of the control group reported not smoking in the past 7 days at 3 months (p<0.01). Biochemical verification of smoking status at 3 months indicated no significant differences between groups (15.60% in the intervention group and 10.93% in the control group [p=0.13]), although significant differences favoring the intervention were found for older smokers (p<0.05) and for those who enrolled in their second or third trimester of pregnancy (p<0.05). Self-report of late pregnancy 7- and 30-day point prevalence abstinence favored the intervention group (p<0.001, p<0.01). No significant differences were observed at the 6-month follow-up or in the postpartum period.
Conclusions: Results provide limited support of the efficacy of the Quit4baby text messaging program in the short term and late in pregnancy, but not in the postpartum period.
Copyright © 2017 American Journal of Preventive Medicine. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Raise the Bar to Help Pregnant Smokers, Rather Than Lower It.Am J Prev Med. 2018 May;54(5):722. doi: 10.1016/j.amepre.2018.01.003. Am J Prev Med. 2018. PMID: 29680120 No abstract available.
References
-
- U.S. DHHS. The health consequences of smoking—50 years of progress: A report of the surgeon general. Atlanta, GA: The Surgeon General; [Accessed December 17, 2016]. www.surgeongeneral.gov/library/reports/50-years-of-progress/. Published 2014.
-
- Pineles BL, Hsu S, Park E, Samet JM. Systematic review and meta-analyses of perinatal death and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol. 2016;184(2):87–97. https://doi.org/10.1093/aje/kwv301. - DOI - PMC - PubMed
-
- Centers for Medicare & Medicaid Services. Tobacco cessation. Washington, DC: Medicaid; www.medicaid.gov/medicaid/quality-of-care/improvement-initiatives/tobacc....
-
- Curtin SC, Matthews TJ. Smoking prevalence and cessation before and during pregnancy: Data from the birth certificate, 2014. Natl Vital Stat Rep. 2016;65(1):1–14. - PubMed
-
- CDC. PRAMstat System: Maternal Behavior/Health, Tobacco Use, 2011. Indicator for whether mother smoked during the last three months of pregnancy
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous