Are survival and mortality rates associated with recruitment to clinical trials in teenage and young adult patients with acute lymphoblastic leukaemia? A retrospective observational analysis in England
- PMID: 28982824
- PMCID: PMC5639992
- DOI: 10.1136/bmjopen-2017-017052
Are survival and mortality rates associated with recruitment to clinical trials in teenage and young adult patients with acute lymphoblastic leukaemia? A retrospective observational analysis in England
Abstract
Objective: Participation rates in clinical trials are low in teenagers and young adults (TYA) with cancer. Whilst the importance of clinical trials in informing best practice is well established, data regarding individual patient benefit are scarce. We have investigated the association between overall survival and trial recruitment in TYA patients with acute lymphoblastic leukaemia (ALL).
Design: Retrospective.
Setting: National (England) TYA patients treated for ALL.
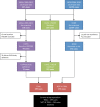
Participants: 511 patients aged 15-24 years diagnosed with ALL between 2004 and 2010 inclusive, of whom 239 (46.7%) participated in the UKALL2003 trial.
Outcome measures: Patients were identified using National Clinical Trial (UKALL2003) and Cancer Registry (National Cancer Data Repository, English National Cancer Online Registration Environment) Databases. Relative survival rates were calculated for trial and non-trial patients and observed differences were modelled using a multiple regression approach. The numbers and percentages of deaths in those patients included in the survival analysis were determined for each 3-month period, p values were calculated using the two-tailed z-test for difference between proportions and 95% CIs for percentage deaths were derived using the binomial distribution based on the Wilson Score method.
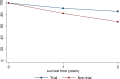
Results: Patients treated on the trial had a 17.9% better 2-year survival (85.4% vs 67.5%, p<0.001) and 8.9% better 1-year survival (90.8% vs 81.9%, p=0.004) than those not on the trial. 35 (14.6%) patients recruited to the trial died in the 2 years following diagnosis compared with 86 (32.6%) of those not recruited (p<0.001).
Conclusions: TYA patients recruited to the clinical trial UKALL 2003 in England had a lower risk of mortality and a higher overall survival than contemporaneous non-trial patients. These data underline the potential for individual patient benefit in participating in a clinical trial and the importance of international efforts to increase trial participation in the TYA age group.
Trial registration number: ISRCTN07355119.
Keywords: acute lymphoblastic leukaemia; clinical trials; survival; teenage and young adult.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources