Pattern of somatic mutations in patients with Waldenström macroglobulinemia or IgM monoclonal gammopathy of undetermined significance
- PMID: 28983055
- PMCID: PMC5709107
- DOI: 10.3324/haematol.2017.172718
Pattern of somatic mutations in patients with Waldenström macroglobulinemia or IgM monoclonal gammopathy of undetermined significance
Abstract
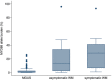
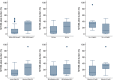
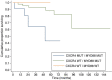
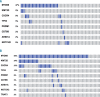
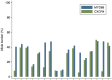
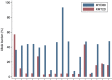
We analyzed MYD88 and CXCR4 mutation status of 260 patients with Waldenström macroglobulinemia or IgM monoclonal gammopathy of undetermined significance using allele-specific real time quantitative polymerase chain reaction and Sanger sequencing, respectively. A subgroup of 119 patients was further studied with next-generation sequencing of 11 target genes (MYD88, CXCR4, ARID1A, KMT2D, NOTCH2, TP53, PRDM1, CD79B, TRAF3, MYBBP1A, and TNFAIP3). MYD88 (L265P) was found at diagnosis in 91% of patients with Waldenström macroglobulinemia and in 60% of patients with IgM monoclonal gammopathy of undetermined significance using allele-specific polymerase chain reaction analysis. MYD88 mutations other than the classical L265P (V217F, S219C and M232T) were found in four cases by next-generation sequencing. Waldenström macroglobulinemia patients with wild-type MYD88 had a distinct clinical phenotype characterized by less bone marrow infiltration (P=0.01) and more frequent extramedullary involvement (P=0.001) compared to patients with mutated MYD88 Patients with wild-type MYD88 did not show additional mutations in the other target genes. CXCR4 mutations were found by Sanger sequencing in 22% of patients with Waldenström macroglobulinemia. With next-generation sequencing, a CXCR4 mutation was detected in 23% of patients with Waldenström macroglobulinemia and 9% of those with IgM monoclonal gammopathy of undetermined significance. Asymptomatic Waldenström macroglobulinemia patients harboring a CXCR4 mutation had a shorter treatment-free survival (51 months) than that of patients with wild-type CXCR4 (median not reached) (P=0.007). Analysis of variant allele frequencies indicated that CXCR4 mutations were present in the dominant clone in the majority of cases. Recurrent somatic mutations of KMT2D were found in 24% of patients with Waldenström macroglobulinemia and 5% of patients with IgM monoclonal gammopathy of undetermined significance and were primarily subclonal.
Copyright© 2017 Ferrata Storti Foundation.
Figures

References
-
- Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30(2):110–115. - PubMed
-
- Treon SP, Hunter ZR, Aggarwal A, et al. Characterization of familial Waldenstrom’s macroglobulinemia. Ann Oncol. 2006;17(3): 488–494. - PubMed
-
- Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med. 2012;367(9): 826–833. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

