Cystic fibrosis swine fail to secrete airway surface liquid in response to inhalation of pathogens
- PMID: 28983075
- PMCID: PMC5629252
- DOI: 10.1038/s41467-017-00835-7
Cystic fibrosis swine fail to secrete airway surface liquid in response to inhalation of pathogens
Abstract
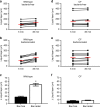
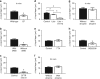
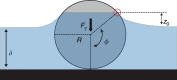
Cystic fibrosis is caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) channel, which can result in chronic lung disease. The sequence of events leading to lung disease is not fully understood but recent data show that the critical pathogenic event is the loss of the ability to clear bacteria due to abnormal airway surface liquid secretion (ASL). However, whether the inhalation of bacteria triggers ASL secretion and whether this is abnormal in cystic fibrosis has never been tested. Here we show, using a novel synchrotron-based in vivo imaging technique, that wild-type pigs display both a basal and a Toll-like receptor-mediated ASL secretory response to the inhalation of cystic fibrosis relevant bacteria. Both mechanisms fail in CFTR-/- swine, suggesting that cystic fibrosis airways do not respond to inhaled pathogens, thus favoring infection and inflammation that may eventually lead to tissue remodeling and respiratory disease.Cystic fibrosis is caused by mutations in the CFTR chloride channel, leading to reduced airway surface liquid secretion. Here the authors show that exposure to bacteria triggers secretion in wild-type but not in pig models of cystic fibrosis, suggesting an impaired response to pathogens contributes to infection.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

