I2-Catalyzed sulfenylation of indoles and pyrroles using triethylammonium thiolates as sulfenylating agents
- PMID: 28983405
- PMCID: PMC5624724
- DOI: 10.1039/C6QO00851H
I2-Catalyzed sulfenylation of indoles and pyrroles using triethylammonium thiolates as sulfenylating agents
Abstract
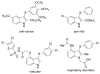
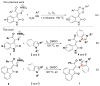
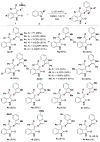
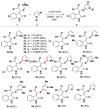
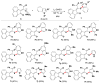
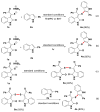
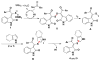
Readily available triethylammonium thiolates were proven to be new and eco-friendly sulfenylating agents for the efficient and practical construction of sulfenylated indoles and pyrroles (48 examples) with good to excellent yields under metal-free and microwave irradiation conditions. The combination of I2 and DMSO enabled direct C-S bond formation, allowing easy and low-cost access to new functionalized C,S-tethered bisindoles and pyrrole-indole pairs with a wide diversity of substituents. The mechanism involving S-S and S-I bond-forming/breaking events was proposed.
Figures
Similar articles
-
I2/O2-Enabled N-S Bond Formation to Access Functionalized 1,2,3-Thiadiazoles.Org Lett. 2016 Mar 18;18(6):1258-61. doi: 10.1021/acs.orglett.6b00079. Epub 2016 Mar 1. Org Lett. 2016. PMID: 26927417
-
Thiosuccinimide enabled S-N bond formation to access N-sulfenylated sulfonamide derivatives with synthetic diversity.Org Biomol Chem. 2024 Jan 31;22(5):990-997. doi: 10.1039/d3ob01848b. Org Biomol Chem. 2024. PMID: 38180390
-
Transition-metal-free KI-catalyzed regioselective sulfenylation of 4-anilinocoumarins using Bunte salts.Org Biomol Chem. 2018 Nov 7;16(43):8015-8019. doi: 10.1039/c8ob02268b. Org Biomol Chem. 2018. PMID: 30334050
-
Pd-catalyzed C-H bond functionalization on the indole and pyrrole nucleus.Top Curr Chem. 2010;292:85-121. doi: 10.1007/128_2009_15. Top Curr Chem. 2010. PMID: 21500404 Review.
-
N-Sulfenylsuccinimide/phthalimide: an alternative sulfenylating reagent in organic transformations.Beilstein J Org Chem. 2023 Sep 27;19:1471-1502. doi: 10.3762/bjoc.19.106. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 37799175 Free PMC article. Review.
References
-
- Sundberg RL. Indoles. Academic; London: 1996.
- Casapullo A, Bifulco G, Bruno I, Riccio RJ. Nat Prod. 2000;63:447. - PubMed
- Humphrey GR, Kuethe JT. Chem Rev. 2006;106:2875. - PubMed
- Katritzky AR, Pozharskii AF. Handbook of Heterocyclic Chemistry. Pergamon; Oxford: 2000.
- Bao B, Sun Q, Yao X, Hong J, Lee CO, Sim CJ, Im KS, Jung JH. J Nat Prod. 2005;68:711. - PubMed
- Cacchi S, Fabrizi G. Chem Rev. 2005;105:2873. - PubMed
- Van Zandt MC, Jones ML, Gunn DE, Geraci LS, Jones JH, Sawicki DR, Sredy J, Jacot JL, Dicioccio AT, Petrova T, Mischler A, Podjarny AD. J Med Chem. 2005;48:3141. - PubMed
- Garbe TR, Kobayashi M, Shimizu N, Takesue N, Ozawa M, Yukawa H. J Nat Prod. 2000;63:596. - PubMed
- Gribble GW. J Chem Soc Perkin Trans 1. 2000:1045.
-
- Ragno R, Coluccia A, La Regina G, De Martino G, Piscitelli F, Lavecchia A, Novellino E, Bergamini A, Ciaprini C, Sinistro A, Maga G, Crespan E, Artico M, Silvestri R. J Med Chem. 2006;49:3172. - PubMed
-
- La Regina G, Edler MC, Brancale A, Kandil S, Coluccia A, Piscitelli F, Hamel E, De Martino G, Matesanz R, Díaz JF, Scovassi AI, Prosperi E, Lavecchia A, Novellino E, Artico M, Silvestri R. J Med Chem. 2007;50:2865. - PubMed
-
- Berger JP, Doebber TW, Leibowitz M, Moller DE, Mosley RT, Tolman RL, Ventre J, Zhang BB, Zhou G. WO 0130343. PCT Int Appl. 2001
- Ramakrishna VSN, Shirsath VS, Kambhampati RS, Vishwakarma S, Kandikere NV, Kota S, Jasti V. WO 2007020653. PCT Int Appl. 2007
-
- Unangst PC, Connor DT, Stabler SR, Weikert RJ, Carethers ME, Kennedy JA, Thueson DO, Chestnut JC, Adolphson RL, Conroy MC. J Med Chem. 1989;32:1360. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
