Iguratimod prevents ovariectomy‑induced bone loss and suppresses osteoclastogenesis via inhibition of peroxisome proliferator‑activated receptor‑γ
- PMID: 28983607
- PMCID: PMC5779905
- DOI: 10.3892/mmr.2017.7648
Iguratimod prevents ovariectomy‑induced bone loss and suppresses osteoclastogenesis via inhibition of peroxisome proliferator‑activated receptor‑γ
Abstract
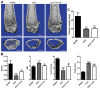
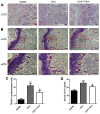
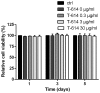
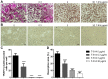
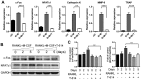
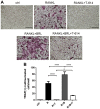
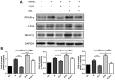
Iguratimod is known for its anti‑inflammatory activities and therapeutic effects in patients with rheumatoid arthritis. It has previously been demonstrated that iguratimod attenuates bone destruction and osteoclast formation in the Walker 256 rat mammary gland carcinoma cell‑induced bone cancer pain model. Therefore, it was hypothesized that iguratimod may additionally exhibit therapeutic effects on benign osteoclast‑associated diseases including postmenopausal osteoporosis. In the present study, ovariectomized mice were used to investigate the effects of iguratimod in vivo. Bone marrow mononuclear cells were cultured to detect the effects of iguratimod on receptor activator of nuclear factor‑κB ligand (RANKL)‑induced osteoclastogenesis in vitro and the molecular mechanisms involved. It was demonstrated that iguratimod may prevent ovariectomy‑induced bone loss by suppressing osteoclast activity in vivo. Consistently, iguratimod may inhibit RANKL‑induced osteoclastogenesis and bone resorption in primary bone marrow mononuclear cells. At the molecular level, peroxisome proliferator‑activated receptor‑γ (PPAR‑γ)/c‑Fos pathway, which is essential in RANKL‑induced osteoclast differentiation, was suppressed by iguratimod. Subsequently, iguratimod decreased the expression of nuclear factor of activated T cells c1 and downstream osteoclast marker genes. The results of the present study demonstrated that iguratimod may inhibit ovariectomy‑induced bone loss and osteoclastogenesis by modulating RANKL signaling. Therefore, iguratimod may act as a novel therapeutic to prevent postmenopausal osteoporosis.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

