Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience
- PMID: 28983798
- PMCID: PMC5656728
- DOI: 10.1007/s12325-017-0612-x
Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience
Abstract
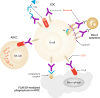
Rituximab is a human/murine, chimeric anti-CD20 monoclonal antibody with established efficacy, and a favorable and well-defined safety profile in patients with various CD20-expressing lymphoid malignancies, including indolent and aggressive forms of B-cell non-Hodgkin lymphoma. Since its first approval 20 years ago, intravenously administered rituximab has revolutionized the treatment of B-cell malignancies and has become a standard component of care for follicular lymphoma, diffuse large B-cell lymphoma, chronic lymphocytic leukemia, and mantle cell lymphoma. For all of these diseases, clinical trials have demonstrated that rituximab not only prolongs the time to disease progression but also extends overall survival. Efficacy benefits have also been shown in patients with marginal zone lymphoma and in more aggressive diseases such as Burkitt lymphoma. Although the proven clinical efficacy and success of rituximab has led to the development of other anti-CD20 monoclonal antibodies in recent years (e.g., obinutuzumab, ofatumumab, veltuzumab, and ocrelizumab), rituximab is likely to maintain a position within the therapeutic armamentarium because it is well established with a long history of successful clinical use. Furthermore, a subcutaneous formulation of the drug has been approved both in the EU and in the USA for the treatment of B-cell malignancies. Using the wealth of data published on rituximab during the last two decades, we review the preclinical development of rituximab and the clinical experience gained in the treatment of hematologic B-cell malignancies, with a focus on the well-established intravenous route of administration. This article is a companion paper to A. Davies, et al., which is also published in this issue.
Funding: F. Hoffmann-La Roche Ltd., Basel, Switzerland.
Keywords: B-cell lymphoma; CD20; Chronic lymphocytic leukemia; Diffuse large B-cell lymphoma; Follicular lymphoma; Monoclonal antibody; Non-Hodgkin lymphoma; Rituximab; Safety; Treatment outcome.
Figures
References
-
- Boffetta P. Epidemiology of adult non-Hodgkin lymphoma. Ann Oncol. 2011;22(Suppl 4):iv27–31.
-
- National Cancer Institute. SEER cancer statistics review 1975–2013. Non-Hodgkin lymphoma. 2016. https://seer.cancer.gov/csr/1975_2013/results_merged/sect_19_nhl.pdf. Accessed 21 Jun 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

