The highs and lows of programmed cardiovascular disease by developmental hypoxia: studies in the chicken embryo
- PMID: 28983923
- PMCID: PMC6068112
- DOI: 10.1113/JP274111
The highs and lows of programmed cardiovascular disease by developmental hypoxia: studies in the chicken embryo
Abstract
It is now established that adverse conditions during pregnancy can trigger a fetal origin of cardiovascular dysfunction and/or increase the risk of heart disease in later life. Suboptimal environmental conditions during early life that may promote the development of cardiovascular dysfunction in the offspring include alterations in fetal oxygenation and nutrition as well as fetal exposure to stress hormones, such as glucocorticoids. There has been growing interest in identifying the partial contributions of each of these stressors to programming of cardiovascular dysfunction. However, in humans and in many animal models this is difficult, as the challenges cannot be disentangled. By using the chicken embryo as an animal model, science has been able to circumvent a number of problems. In contrast to mammals, in the chicken embryo the effects on the developing cardiovascular system of changes in oxygenation, nutrition or stress hormones can be isolated and determined directly, independent of changes in the maternal or placental physiology. In this review, we summarise studies that have exploited the chicken embryo model to determine the effects on prenatal growth, cardiovascular development and pituitary-adrenal function of isolated chronic developmental hypoxia.
Keywords: IUGR; cardiovascular disease; fetus; hypoxia; programming.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures
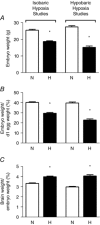

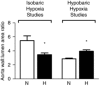
Similar articles
-
Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis--2012 Curt Richter Award Winner.Psychoneuroendocrinology. 2013 Jan;38(1):1-11. doi: 10.1016/j.psyneuen.2012.08.012. Epub 2012 Sep 19. Psychoneuroendocrinology. 2013. PMID: 22998948 Review.
-
Epigenetic regulation by hypoxia, N-acetylcysteine and hydrogen sulphide of the fetal vasculature in growth restricted offspring: A study in humans and chicken embryos.J Physiol. 2024 Aug;602(15):3833-3852. doi: 10.1113/JP286266. Epub 2024 Jul 10. J Physiol. 2024. PMID: 38985827
-
Sildenafil therapy for fetal cardiovascular dysfunction during hypoxic development: studies in the chick embryo.J Physiol. 2017 Mar 1;595(5):1563-1573. doi: 10.1113/JP273393. Epub 2016 Dec 11. J Physiol. 2017. PMID: 27861916 Free PMC article.
-
Early-life glucocorticoid exposure: the hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk.Endocr Rev. 2013 Dec;34(6):885-916. doi: 10.1210/er.2013-1012. Epub 2013 Aug 22. Endocr Rev. 2013. PMID: 23970762 Review.
-
Glucocorticoids and fetal programming part 1: Outcomes.Nat Rev Endocrinol. 2014 Jul;10(7):391-402. doi: 10.1038/nrendo.2014.73. Epub 2014 May 27. Nat Rev Endocrinol. 2014. PMID: 24863382 Review.
Cited by
-
Altered Cardiovascular Defense to Hypotensive Stress in the Chronically Hypoxic Fetus.Hypertension. 2020 Oct;76(4):1195-1207. doi: 10.1161/HYPERTENSIONAHA.120.15384. Epub 2020 Aug 31. Hypertension. 2020. PMID: 32862711 Free PMC article.
-
Isolating adverse effects of glucocorticoids on the embryonic cardiovascular system.FASEB J. 2020 Jul;34(7):9664-9677. doi: 10.1096/fj.202000697R. Epub 2020 Jun 5. FASEB J. 2020. PMID: 32502311 Free PMC article.
-
Advances in cellular and integrative control of oxygen and carbon dioxide homeostasis.J Physiol. 2018 Aug;596(15):2933-2934. doi: 10.1113/JP276326. J Physiol. 2018. PMID: 30239019 Free PMC article. No abstract available.
-
Developmental plasticity of the cardiovascular system in oviparous vertebrates: effects of chronic hypoxia and interactive stressors in the context of climate change.J Exp Biol. 2024 Oct 15;227(20):jeb245530. doi: 10.1242/jeb.245530. Epub 2024 Aug 7. J Exp Biol. 2024. PMID: 39109475 Free PMC article. Review.
-
Oxidative Stress-Induced Hypertension of Developmental Origins: Preventive Aspects of Antioxidant Therapy.Antioxidants (Basel). 2022 Mar 7;11(3):511. doi: 10.3390/antiox11030511. Antioxidants (Basel). 2022. PMID: 35326161 Free PMC article. Review.
References
-
- Agren P, van der Sterren S, Cogolludo AL, Frazziano G, de Mey JG, Blanco CE & Villamor E (2008). Developmental changes in endothelium‐dependent relaxation of the chicken ductus arteriosus. J Physiol Pharmacol 59, 55–76. - PubMed
-
- Aiken CE & Ozanne SE (2013). Sex differences in developmental programming models. Reproduction 145, R1–R13. - PubMed
-
- Akira M & Yoshiyuki S (2006). Placental circulation, fetal growth, and stiffness of the abdominal aorta in newborn infants. J Pediatr 148, 49–53. - PubMed
-
- Arnett DK, Evans GW & Riley WA (1994). Arterial stiffness: a new cardiovascular risk factor? Am J Epidemiol 140, 669–682. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

