Mechanisms of carbon nanotube-induced pulmonary fibrosis: a physicochemical characteristic perspective
- PMID: 28984415
- PMCID: PMC5977978
- DOI: 10.1002/wnan.1498
Mechanisms of carbon nanotube-induced pulmonary fibrosis: a physicochemical characteristic perspective
Abstract
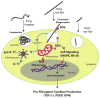
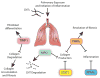
Carbon nanotubes (CNTs) are engineered nanomaterials (ENMs) with numerous beneficial applications. However, they could pose a risk to human health from occupational or consumer exposures. Rodent models demonstrate that exposure to CNTs via inhalation, instillation, or aspiration results in pulmonary fibrosis. The severity of the fibrogenic response is determined by various physicochemical properties of the nanomaterial such as residual metal catalyst content, rigidity, length, aggregation status, or surface charge. CNTs are also increasingly functionalized post-synthesis with organic or inorganic agents to modify or enhance surface properties. The mechanisms of CNT-induced fibrosis involve oxidative stress, innate immune responses of macrophages, cytokine and growth factor production, epithelial cell injury and death, expansion of the pulmonary myofibroblast population, and consequent extracellular matrix accumulation. A comprehensive understanding of how physicochemical properties affect the fibrogenic potential of various types of CNTs should be considered in combination with genetic variability and gain or loss of function of specific genes encoding secreted cytokines, enzymes, or intracellular cell signaling molecules. Here, we cover the current state of the literature on mechanisms of CNT-exposed pulmonary fibrosis in rodent models with a focus on physicochemical characteristics as principal drivers of the mechanisms leading to pulmonary fibrosis. This article is categorized under: Therapeutic Approaches and Drug Discovery > Nanomedicine for Respiratory Disease Toxicology and Regulatory Issues in Nanomedicine > Toxicology of Nanomaterials.
© 2017 Wiley Periodicals, Inc.
Figures

References
-
- The National Institute for Occupational Safety and Health (NIOSH) Occupational Exposure to Carbon Nanotubes and Nanofibers. Current Intelligence Bulletin. 2013;65:1–156.
-
- Pauluhn J. Subchronic 13-Week Inhalation Exposure of Rats to Multiwalled Carbon Nanotubes: Toxic Effects Are Determined by Density of Agglomerate Structures, Not Fibrillar Structures. Toxicol Sci. 2010;113:226–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

