TGF-β1 Evokes Human Airway Smooth Muscle Cell Shortening and Hyperresponsiveness via Smad3
- PMID: 28984468
- PMCID: PMC5946330
- DOI: 10.1165/rcmb.2017-0247OC
TGF-β1 Evokes Human Airway Smooth Muscle Cell Shortening and Hyperresponsiveness via Smad3
Abstract
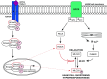
Transforming growth factor β1 (TGF-β1), a cytokine whose levels are elevated in the airways of patients with asthma, perpetuates airway inflammation and modulates airway structural cell remodeling. However, the role of TGF-β1 in excessive airway narrowing in asthma, or airway hyperresponsiveness (AHR), remains unclear. In this study, we set out to investigate the direct effects of TGF-β1 on human airway smooth muscle (HASM) cell shortening and hyperresponsiveness. The dynamics of AHR and single-cell excitation-contraction coupling were measured in human precision-cut lung slices and in isolated HASM cells using supravital microscopy and magnetic twisting cytometry, respectively. In human precision-cut lung slices, overnight treatment with TGF-β1 significantly augmented basal and carbachol-induced bronchoconstriction. In isolated HASM cells, TGF-β1 increased basal and methacholine-induced cytoskeletal stiffness in a dose- and time-dependent manner. TGF-β1-induced single-cell contraction was corroborated by concomitant increases in myosin light chain and myosin phosphatase target subunit 1 phosphorylation levels, which were attenuated by small interfering RNA-mediated knockdown of Smad3 and pharmacological inhibition of Rho kinase. Strikingly, these physiological effects of TGF-β1 occurred through a RhoA-independent mechanism, with little effect on HASM cell [Ca2+]i levels. Together, our data suggest that TGF-β1 enhances HASM excitation-contraction coupling pathways to induce HASM cell shortening and hyperresponsiveness. These findings reveal a potential link between airway injury-repair responses and bronchial hyperreactivity in asthma, and define TGF-β1 signaling as a potential target to reduce AHR in asthma.
Keywords: asthma; bronchoconstriction; contraction; cytokines; remodeling.
Figures
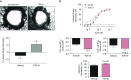
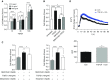

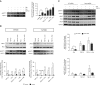

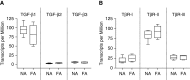
Comment in
-
Does Transforming Growth Factor-β Induce Persistent Airway Obstruction after Asthma Exacerbations?Am J Respir Cell Mol Biol. 2018 May;58(5):543-544. doi: 10.1165/rcmb.2017-0339ED. Am J Respir Cell Mol Biol. 2018. PMID: 29714630 No abstract available.
References
-
- McDonald VM, Maltby S, Reddel HK, King GG, Wark PAB, Smith L, et al. Severe asthma: current management, targeted therapies and future directions—a roundtable report. 2017;22:53–60. - PubMed
-
- Hekking P-PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. 2015;135:896–902. - PubMed
-
- Yang Y, Zhang N, Lan F, Van Crombruggen K, Fang L, Hu G, et al. Transforming growth factor-β1 pathways in inflammatory airway diseases. 2014;69:699–707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

