Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 0539
- PMID: 28984517
- PMCID: PMC5889346
- DOI: 10.3171/2016.11.JNS161170
Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 0539
Erratum in
-
Erratum. Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 0539.J Neurosurg. 2018 Dec 1;129(6):1650. doi: 10.3171/2018.8.JNS161170a. Epub 2018 Sep 28. J Neurosurg. 2018. PMID: 30485244 No abstract available.
Abstract
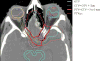
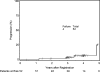
OBJECTIVE This is the first clinical outcomes report of NRG Oncology RTOG 0539, detailing the primary endpoint, 3-year progression-free survival (PFS), compared with a predefined historical control for intermediate-risk meningioma, and secondarily evaluating overall survival (OS), local failure, and prospectively scored adverse events (AEs). METHODS NRG Oncology RTOG 0539 was a Phase II clinical trial allocating meningioma patients to 1 of 3 prognostic groups and management strategies according to WHO grade, recurrence status, and resection extent. For the intermediate-risk group (Group 2), eligible patients had either newly diagnosed WHO Grade II meningioma that had been treated with gross-total resection (GTR; Simpson Grades I-III) or recurrent WHO Grade I meningioma with any resection extent. Pathology and imaging were centrally reviewed. Patients were treated with radiation therapy (RT), either intensity modulated (IMRT) or 3D conformal (3DCRT), 54 Gy in 30 fractions. The RT target volume was defined as the tumor bed and any nodular enhancement (e.g., in patients with recurrent WHO Grade I tumors) with a minimum 8-mm and maximum 15-mm margin, depending on tumor location and setup reproducibility of the RT method. The primary endpoint was 3-year PFS. Results were compared with historical controls (3-year PFS: 70% following GTR alone and 90% with GTR + RT). AEs were scored using NCI Common Toxicity Criteria. RESULTS Fifty-six patients enrolled in the intermediate-risk group, of whom 3 were ineligible and 1 did not receive RT. Of the 52 patients who received protocol therapy, 4 withdrew without a recurrence before 3 years leaving 48 patients evaluable for the primary endpoint, 3-year PFS, which was actuarially 93.8% (p = 0.0003). Within 3 years, 3 patients experienced events affecting PFS: 1 patient with a WHO Grade II tumor died of the disease, 1 patient with a WHO Grade II tumor had disease progression but remained alive, and 1 patient with recurrent WHO Grade I meningioma died of undetermined cause without tumor progression. The 3-year actuarial local failure rate was 4.1%, and the 3-year OS rate was 96%. After 3 years, progression occurred in 2 additional patients: 1 patient with recurrent WHO Grade I meningioma and 1 patient with WHO Grade II disease; both remain alive. Among 52 evaluable patients who received protocol treatment, 36 (69.2%) had WHO Grade II tumors and underwent GTR, and 16 (30.8%) had recurrent WHO Grade I tumors. There was no significant difference in PFS between these subgroups (p = 0.52, HR 0.56, 95% CI 0.09-3.35), validating their consolidation. Of the 52 evaluable patients, 44 (84.6%) received IMRT, and 50 (96.2%) were treated per protocol or with acceptable variation. AEs (definitely, probably, or possibly related to protocol treatment) were limited to Grade 1 or 2, with no reported Grade 3 events. CONCLUSIONS This is the first clinical outcomes report from NRG Oncology RTOG 0539. Patients with intermediate-risk meningioma treated with RT had excellent 3-year PFS, with a low rate of local failure and a low risk of AEs. These results support the use of postoperative RT for newly diagnosed gross-totally resected WHO Grade II or recurrent WHO Grade I meningioma irrespective of resection extent. They also document minimal toxicity and high rates of tumor control with IMRT. Clinical trial registration no.: NCT00895622 (clinicaltrials.gov).
Keywords: 3DCRT = 3D conformal RT; AE = adverse event; CGE = cobalt gray equivalent; CNED = continual no evidence of disease; CTV = clinical tumor volume; EBRT = external beam RT; EORTC = European Organisation for Research and Treatment of Cancer; GTR = gross-total resection; GTV = gross tumor volume; IMRT = intensity-modulated RT; MMSE = Mini-Mental State Examination; OAR = organ at risk; OS = overall survival; PD = progressive disease; PFS = progression-free survival; PRV = planning risk volume; PTV = planning target volume; PTVPRV = overlap between the PTV and the particular PRV of concern; RT = radiation therapy; RTOG = Radiation Therapy Oncology Group; SD = stable disease; SRS = stereotactic radiosurgery; STR = subtotal resection; WHO = World Health Organization; WHO Grade II (atypical); meningioma; oncology; radiotherapy.
Figures





Comment in
-
Letter to the Editor. Phase III randomized controlled trials are essential to properly evaluate the role of radiotherapy in WHO grade II meningioma.J Neurosurg. 2018 Oct;129(4):1104-1105. doi: 10.3171/2018.6.JNS181418. Epub 2018 Aug 17. J Neurosurg. 2018. PMID: 30117772 No abstract available.
-
Letter to the Editor. Intermediate-risk meningioma and NRG Oncology RTOG 0539.J Neurosurg. 2018 Dec 1;129(6):1651-1653. doi: 10.3171/2018.4.JNS18811. Epub 2018 Sep 28. J Neurosurg. 2018. PMID: 30265197 No abstract available.
References
-
- Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64:56–60. - PubMed
-
- Bagshaw HP, Jensen RL, Palmer CA, Shrieve DC. Stereotactic Radiation Therapy and the Management of Atypical Meningiomas: Outcomes in the Upfront and Recurrent Setting. Int J Radiat Oncol Biol Phys. 2015;93 Abstract.
-
- Boskos C, Feuvret L, Noel G, Habrand JL, Pommier P, Alapetite C, et al. Combined proton and photon conformal radiotherapy for intracranial atypical and malignant meningioma. Int J Radiat Oncol Biol Phys. 2009;75:399–406. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

