Oxidative stress promotes SIRT1 recruitment to the GADD34/PP1α complex to activate its deacetylase function
- PMID: 28984870
- PMCID: PMC5762841
- DOI: 10.1038/cdd.2017.152
Oxidative stress promotes SIRT1 recruitment to the GADD34/PP1α complex to activate its deacetylase function
Abstract
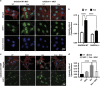
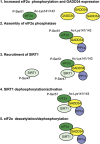
Phosphorylation of the eukaryotic translation initiation factor, eIF2α, by stress-activated protein kinases and dephosphorylation by the growth arrest and DNA damage-inducible protein (GADD34)-containing phosphatase is a central node in the integrated stress response. Mass spectrometry demonstrated GADD34 acetylation at multiple lysines. Substituting K315 and K322 with alanines or glutamines did not impair GADD34's ability to recruit protein phosphatase 1α (PP1α) or eIF2α, suggesting that GADD34 acetylation did not modulate eIF2α phosphatase activity. Arsenite (Ars)-induced oxidative stress increased cellular GADD34 levels and enhanced Sirtuin 1 (SIRT1) recruitment to assemble a cytoplasmic complex containing GADD34, PP1α, eIF2α and SIRT1. Induction of GADD34 in WT MEFs paralleled the dephosphorylation of eIF2α (phosphoserine-51) and SIRT1 (phosphoserine-47). By comparison, eIF2α and SIRT1 were persistently phosphorylated in Ars-treated GADD34-/- MEFs. Expressing WT GADD34, but not a mutant unable to bind PP1α in GADD34-/- MEFs restored both eIF2α and SIRT1 dephosphorylation. SIRT1 dephosphorylation increased its deacetylase activity, measured in vitro and in cells. Loss of function of GADD34 or SIRT1 enhanced cellular p-eIF2α levels and attenuated cell death following Ars exposure. These results highlighted a novel role for the GADD34/PP1α complex in coordinating the dephosphorylation and reactivation of eIF2α and SIRT1 to determine cell fate following oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016; 529: 326–335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

