Clinical Outcomes by Race and Ethnicity in the Systolic Blood Pressure Intervention Trial (SPRINT): A Randomized Clinical Trial
- PMID: 28985268
- PMCID: PMC5861531
- DOI: 10.1093/ajh/hpx138
Clinical Outcomes by Race and Ethnicity in the Systolic Blood Pressure Intervention Trial (SPRINT): A Randomized Clinical Trial
Abstract
Background: The Systolic Blood Pressure Intervention Trial (SPRINT) showed that targeting a systolic blood pressure (SBP) of ≤ 120 mm Hg (intensive treatment) reduced cardiovascular disease (CVD) events compared to SBP of ≤ 140 mm Hg (standard treatment); however, it is unclear if this effect is similar in all racial/ethnic groups.
Methods: We analyzed SPRINT data within non-Hispanic White (NHW), non-Hispanic Black (NHB), and Hispanic subgroups to address this question. High-risk nondiabetic hypertensive patients (N = 9,361; 30% NHB; 11% Hispanic) 50 years and older were randomly assigned to intensive or standard treatment. Primary outcome was a composite of the first occurrence of a myocardial infarction, acute coronary syndrome, stroke, decompensated heart failure, or CVD death.
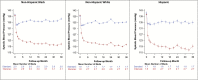
Results: Average postbaseline SBP was similar among NHW, NHB, and Hispanics in both treatment arms. Hazard ratios (HRs) (95% confidence interval) (intensive vs. standard treatment groups) for primary outcome were 0.70 (0.57-0.86), 0.71 (0.51-0.98), 0.62 (0.33-1.15) (interaction P value = 0.85) in NHW, NHB, and Hispanics. CVD mortality HRs were 0.49 (0.29-0.81), 0.77 (0.37-1.57), and 0.17 (0.01-1.08). All-cause mortality HRs were 0.61 (0.47-0.80), 0.92 (0.63-1.35), and 1.58 (0.73-3.62), respectively. A test for differences among racial/ethnic groups in the effect of treatment assignment on all-cause mortality was not significant (Hommel-adjusted P value = 0.062) after adjustment for multiple comparisons.
Conclusion: Targeting a SBP goal of ≤ 120 mm Hg compared to ≤ 140 mm Hg led to similar SBP control and was associated with similar benefits and risks among all racial ethnic groups, though NHBs required an average of ~0.3 more medications.
Clinical trials registration: Trial Number NCT01206062, ClinicalTrials.gov Identifier at https://clinicaltrials.gov/ct2/show/NCT01206062.
Keywords: African Americans; Hispanics; blood pressure; clinical outcomes; clinical trials; hypertension; race and ethnicity.
© The Author 2017. Published by Oxford University Press on behalf of American Journal of Hypertension, Ltd.
Figures
References
-
- Whelton PK, Einhorn PT, Muntner P, Appel LJ, Cushman WC, Diez Roux AV, Ferdinand KC, Rahman M, Taylor HA, Ard J, Arnett DK, Carter BL, Davis BR, Freedman BI, Cooper LA, Cooper R, Desvigne-Nickens P, Gavini N, Go AS, Hyman DJ, Kimmel PL, Margolis KL, Miller ER, 3rd, Mills KT, Mensah GA, Navar AM, Ogedegbe G, Rakotz MK, Thomas G, Tobin JN, Wright JT, Yoon SS, Cutler JA; National Heart, Lung, and Blood Institute Working Group on Research Needs to Improve Hypertension Treatment and Control in African Americans Research needs to improve hypertension treatment and control in African Americans. Hypertension 2016; 68:1066–1072. - PMC - PubMed
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016; 133:e38–360. - PubMed
-
- Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the united states: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 2013; 1–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR000433/TR/NCATS NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- UL1 TR000075/TR/NCATS NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- HHSN268200900048C/HL/NHLBI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- HHSN268200900040C/HL/NHLBI NIH HHS/United States
- HHSN268200900046C/HL/NHLBI NIH HHS/United States
- P30 GM103337/GM/NIGMS NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- HHSN268200900049C/HL/NHLBI NIH HHS/United States
- HHSN268200900047C/HL/NHLBI NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000073/TR/NCATS NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- UL1 TR002003/TR/NCATS NIH HHS/United States
- UL1 TR000002/TR/NCATS NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- UL1 TR000105/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

