KV channels and the regulation of vascular smooth muscle tone
- PMID: 28985443
- PMCID: PMC5760307
- DOI: 10.1111/micc.12421
KV channels and the regulation of vascular smooth muscle tone
Abstract
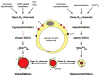
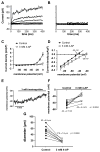
VSMCs in resistance arteries and arterioles express a diverse array of KV channels with members of the KV 1, KV 2 and KV 7 families being particularly important. Members of the KV channel family: (i) are highly expressed in VSMCs; (ii) are active at the resting membrane potential of VSMCs in vivo (-45 to -30 mV); (iii) contribute to the negative feedback regulation of VSMC membrane potential and myogenic tone; (iv) are activated by cAMP-related vasodilators, hydrogen sulfide and hydrogen peroxide; (v) are inhibited by increases in intracellular Ca2+ and vasoconstrictors that signal through Gq -coupled receptors; (vi) are involved in the proliferative phenotype of VSMCs; and (vii) are modulated by diseases such as hypertension, obesity, the metabolic syndrome and diabetes. Thus, KV channels participate in every aspect of the regulation of VSMC function in both health and disease.
Keywords: KV channels; arterioles; blood flow; microcirculation; potassium channels; resistance arteries; vascular smooth muscle; vasoconstriction; vasodilation.
© 2017 John Wiley & Sons Ltd.
Figures
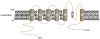
References
-
- Aiello EA, Clement-Chomienne O, Sontag DP, Walsh MP, Cole WC. Protein kinase C inhibits delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1996;271:H109–119. - PubMed
-
- Aiello EA, Malcolm AT, Walsh MP, Cole WC. Beta-adrenoceptor activation and PKA regulate delayed rectifier K+ channels of vascular smooth muscle cells. American Journal of Physiology. 1998;275:H448–H459. - PubMed
-
- Aiello EA, Walsh MP, Cole WC. Isoproterenol and forskolin increase and PKI inhibits delayed rectifier K+ current in vascular myocytes isolated from rabbit coronary artery and portal vein. CanJPhysiolPharmacol. 1994;72:47.
-
- Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. AmJPhysiolHeart CircPhysiol. 1995;268:H926–H934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

