Lactate Metabolism in Human Lung Tumors
- PMID: 28985563
- PMCID: PMC5684706
- DOI: 10.1016/j.cell.2017.09.019
Lactate Metabolism in Human Lung Tumors
Abstract
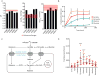
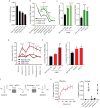
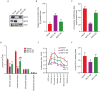
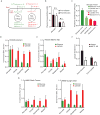
Cancer cells consume glucose and secrete lactate in culture. It is unknown whether lactate contributes to energy metabolism in living tumors. We previously reported that human non-small-cell lung cancers (NSCLCs) oxidize glucose in the tricarboxylic acid (TCA) cycle. Here, we show that lactate is also a TCA cycle carbon source for NSCLC. In human NSCLC, evidence of lactate utilization was most apparent in tumors with high 18fluorodeoxyglucose uptake and aggressive oncological behavior. Infusing human NSCLC patients with 13C-lactate revealed extensive labeling of TCA cycle metabolites. In mice, deleting monocarboxylate transporter-1 (MCT1) from tumor cells eliminated lactate-dependent metabolite labeling, confirming tumor-cell-autonomous lactate uptake. Strikingly, directly comparing lactate and glucose metabolism in vivo indicated that lactate's contribution to the TCA cycle predominates. The data indicate that tumors, including bona fide human NSCLC, can use lactate as a fuel in vivo.
Keywords: Cancer metabolism; Glycolysis; Lactate; Lung cancer; Metabolic flux analysis; Monocarboxylate transport; Tricarboxylic Acid Cycle; Warburg effect.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Metabolism: More lactate, please.Nat Rev Cancer. 2017 Dec;17(12):707. doi: 10.1038/nrc.2017.101. Epub 2017 Oct 27. Nat Rev Cancer. 2017. PMID: 29077692 No abstract available.
-
Waste Not, Want Not: Lactate Oxidation Fuels the TCA Cycle.Cell Metab. 2017 Dec 5;26(6):803-804. doi: 10.1016/j.cmet.2017.11.005. Cell Metab. 2017. PMID: 29211977
References
-
- Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, Scherpereel A, Mascaux C, Moreau M, Roelandts M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12. - PubMed
-
- Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UE, et al. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell cycle (Georgetown, Tex) 2010;9:3506–3514. - PMC - PubMed
-
- Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016;24:657–671. - PubMed
-
- Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. 2002;30:258–264. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

