Contraception after medication abortion in the United States: results from a cluster randomized trial
- PMID: 28986072
- PMCID: PMC5997454
- DOI: 10.1016/j.ajog.2017.09.020
Contraception after medication abortion in the United States: results from a cluster randomized trial
Abstract
Background: Understanding how contraceptive choices and access differ for women having medication abortions compared to aspiration procedures can help to identify priorities for improved patient-centered postabortion contraceptive care.
Objective: The objective of this study was to investigate the differences in contraceptive counseling, method choices, and use between medication and aspiration abortion patients.
Study design: This subanalysis examines data from 643 abortion patients from 17 reproductive health centers in a cluster, randomized trial across the United States. We recruited participants aged 18-25 years who did not desire pregnancy and followed them for 1 year. We measured the effect of a full-staff contraceptive training and abortion type on contraceptive counseling, choice, and use with multivariable regression models, using generalized estimating equations for clustering. We used survival analysis with shared frailty to model actual intrauterine device and subdermal implant initiation over 1 year.
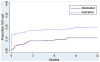
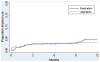
Results: Overall, 26% of participants (n = 166) had a medication abortion and 74% (n = 477) had an aspiration abortion at the enrollment visit. Women obtaining medication abortions were as likely as those having aspiration abortions to receive counseling on intrauterine devices or the implant (55%) and on a short-acting hormonal method (79%). The proportions of women choosing to use these methods (29% intrauterine device or implant, 58% short-acting hormonal) were also similar by abortion type. The proportions of women who actually used short-acting hormonal methods (71% medication vs 57% aspiration) and condoms or no method (20% vs 22%) within 3 months were not significantly different by abortion type. However, intrauterine device initiation over a year was significantly lower after the medication than the aspiration abortion (11 per 100 person-years vs 20 per 100 person-years, adjusted hazard ratio, 0.50; 95% confidence interval, 0.28-0.89). Implant initiation rates were low and similar by abortion type (5 per 100 person-years vs 4 per 100 person-years, adjusted hazard ratio, 2.41; 95% confidence interval, 0.88-6.59). In contrast to women choosing short-acting methods, relatively few of those choosing a long-acting method at enrollment, 34% of medication abortion patients and 53% of aspiration abortion patients, had one placed within 3 months. Neither differences in health insurance nor pelvic examination preferences by abortion type accounted for lower intrauterine device use among medication abortion patients.
Conclusion: Despite similar contraceptive choices, fewer patients receiving medication abortion than aspiration abortion initiated intrauterine devices over 1 year of follow-up. Interventions to help patients receiving medication abortion to successfully return for intrauterine device placement are warranted. New protocols for same-day implant placement may also help patients receiving medication abortion and desiring a long-acting method to receive one.
Trial registration: ClinicalTrials.gov NCT01360216.
Keywords: abortion; implant; intrauterine device; long-acting reversible contraception; medical abortion; medication abortion; postabortion contraception; randomized trial.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr Cadwallader is a Nexplanon trainer (Merck). The other authors report no conflicts of interest.
Figures
Similar articles
-
Funding policies and postabortion long-acting reversible contraception: results from a cluster randomized trial.Am J Obstet Gynecol. 2016 Jun;214(6):716.e1-8. doi: 10.1016/j.ajog.2015.12.009. Epub 2015 Dec 12. Am J Obstet Gynecol. 2016. PMID: 26692178 Free PMC article. Clinical Trial.
-
Contraceptive discontinuation and pregnancy postabortion in Nepal: a longitudinal cohort study.Contraception. 2015 Apr;91(4):301-7. doi: 10.1016/j.contraception.2014.12.011. Epub 2014 Dec 30. Contraception. 2015. PMID: 25553872
-
Immediate postabortion initiation of levonorgestrel implants reduces the incidence of births and abortions at 2 years and beyond.Contraception. 2015 Jul;92(1):17-25. doi: 10.1016/j.contraception.2015.03.012. Epub 2015 Mar 27. Contraception. 2015. PMID: 25818594
-
Long-acting reversible contraception immediately after medical abortion: systematic review with meta-analyses.Hum Reprod Update. 2020 Feb 28;26(2):141-160. doi: 10.1093/humupd/dmz040. Hum Reprod Update. 2020. PMID: 32096862
-
Canadian Contraception Consensus (Part 1 of 4).J Obstet Gynaecol Can. 2015 Oct;37(10):936-42. doi: 10.1016/s1701-2163(16)30033-0. J Obstet Gynaecol Can. 2015. PMID: 26606712 English, French.
Cited by
-
Provision of long-acting reversible contraception at surgical abortion-A cross-sectional nationwide register study.Acta Obstet Gynecol Scand. 2022 Jan;101(1):77-83. doi: 10.1111/aogs.14289. Epub 2021 Nov 10. Acta Obstet Gynecol Scand. 2022. PMID: 34761384 Free PMC article.
-
Long-acting reversible contraception initiation after medication abortion: a retrospective cohort study.Contracept Reprod Med. 2025 May 28;10(1):34. doi: 10.1186/s40834-025-00371-6. Contracept Reprod Med. 2025. PMID: 40437647 Free PMC article.
References
-
- Cameron S, Glasier A, Dewart H, Johnstone A. Women’s experiences of the final stage of early medical abortion at home: results of a pilot survey. J Fam Plan Reprod Health Care. 2010;36:213–6. - PubMed
-
- Finer LB, Wei J. Effect of mifepristone on abortion access in the United States. Obstet Gynecol. 2009;114:623–30. - PubMed
-
- Centers for Disease Control and Prevention, Department of Health and Human Services. US medical eligibility criteria for contraceptive use, 2010. MMWR Morb Mort Wkly Rep. 2010;59:2.
-
- Grimes D, Schulz K, Stanwood N. Immediate postabortal insertion of intrauterine devices. Cochrane Database Syst Rev. 2004:CD001777. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

