Reduced Cardiac Calcineurin Expression Mimics Long-Term Hypoxia-Induced Heart Defects in Drosophila
- PMID: 28986453
- PMCID: PMC5669044
- DOI: 10.1161/CIRCGENETICS.117.001706
Reduced Cardiac Calcineurin Expression Mimics Long-Term Hypoxia-Induced Heart Defects in Drosophila
Erratum in
-
Correction.Circ Cardiovasc Genet. 2017 Dec;10(6):e000041. doi: 10.1161/HCG.0000000000000041. Circ Cardiovasc Genet. 2017. PMID: 30576608 No abstract available.
Abstract
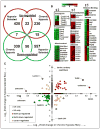
Background: Hypoxia is often associated with cardiopulmonary diseases, which represent some of the leading causes of mortality worldwide. Long-term hypoxia exposures, whether from disease or environmental condition, can cause cardiomyopathy and lead to heart failure. Indeed, hypoxia-induced heart failure is a hallmark feature of chronic mountain sickness in maladapted populations living at high altitude. In a previously established Drosophila heart model for long-term hypoxia exposure, we found that hypoxia caused heart dysfunction. Calcineurin is known to be critical in cardiac hypertrophy under normoxia, but its role in the heart under hypoxia is poorly understood.
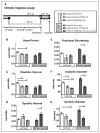
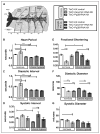
Methods and results: In the present study, we explore the function of calcineurin, a gene candidate we found downregulated in the Drosophila heart after lifetime and multigenerational hypoxia exposure. We examined the roles of 2 homologs of Calcineurin A, CanA14F, and Pp2B in the Drosophila cardiac response to long-term hypoxia. We found that knockdown of these calcineurin catalytic subunits caused cardiac restriction under normoxia that are further aggravated under hypoxia. Conversely, cardiac overexpression of Pp2B under hypoxia was lethal, suggesting that a hypertrophic signal in the presence of insufficient oxygen supply is deleterious.
Conclusions: Our results suggest a key role for calcineurin in cardiac remodeling during long-term hypoxia with implications for diseases of chronic hypoxia, and it likely contributes to mechanisms underlying these disease states.
Keywords: CanA; cardiac remodeling; cardiomyopathy; chronic ischemic heart disease; chronic mountain sickness; hypertrophy; hypoxia adaptation.
© 2017 American Heart Association, Inc.
Figures

Comment in
-
High Heart: A Role for Calcineurin Signaling in Hypoxia-Influenced Cardiac Growth.Circ Cardiovasc Genet. 2017 Oct;10(5):e001919. doi: 10.1161/CIRCGENETICS.117.001919. Circ Cardiovasc Genet. 2017. PMID: 28986459 Free PMC article. No abstract available.
References
-
- Monge C, León-Velarde F. Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol Rev. 1991;71:1135–1172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

