Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration
- PMID: 28986561
- PMCID: PMC5630615
- DOI: 10.1038/s41467-017-00925-6
Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration
Abstract
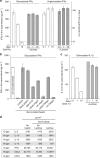
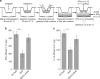
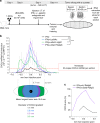
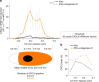
The presence of T cells in tumors predicts overall survival for cancer patients. However, why most tumors are poorly infiltrated by T cells is barely understood. T-cell recruitment towards the tumor requires a chemokine gradient of the critical IFNγ-induced chemokines CXCL9/10/11. Here, we describe how tumors can abolish IFNγ-induced chemokines, thereby reducing T-cell attraction. This mechanism requires extracellular galectin-3, a lectin secreted by tumors. Galectins bind the glycans of glycoproteins and form lattices by oligomerization. We demonstrate that galectin-3 binds the glycans of the extracellular matrix and those decorating IFNγ. In mice bearing human tumors, galectin-3 reduces IFNγ diffusion through the tumor matrix. Galectin antagonists increase intratumoral IFNγ diffusion, CXCL9 gradient and tumor recruitment of adoptively transferred human CD8+ T cells specific for a tumor antigen. Transfer of T cells reduces tumor growth only if galectin antagonists are injected. Considering that most human cytokines are glycosylated, galectin secretion could be a general strategy for tumor immune evasion.Most tumours are poorly infiltrated by T cells. Here the authors show that galectin-3 secreted by tumours binds both glycosylated IFNγ and glycoproteins of the tumour extracellular matrix, thus avoiding IFNγ diffusion and the formation of an IFNγ-induced chemokine gradient required for T cell infiltration.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

