The drinking water contaminant dibromoacetonitrile delays G1-S transition and suppresses Chk1 activation at broken replication forks
- PMID: 28986587
- PMCID: PMC5630572
- DOI: 10.1038/s41598-017-13033-8
The drinking water contaminant dibromoacetonitrile delays G1-S transition and suppresses Chk1 activation at broken replication forks
Abstract
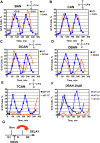
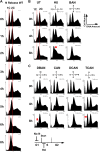
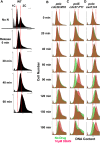
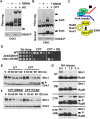
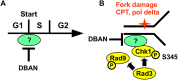
Chlorination of drinking water protects humans from water-born pathogens, but it also produces low concentrations of dibromoacetonitrile (DBAN), a common disinfectant by-product found in many water supply systems. DBAN is not mutagenic but causes DNA breaks and elevates sister chromatid exchange in mammalian cells. The WHO issued guidelines for DBAN after it was linked with cancer of the liver and stomach in rodents. How this haloacetonitrile promotes malignant cell transformation is unknown. Using fission yeast as a model, we report here that DBAN delays G1-S transition. DBAN does not hinder ongoing DNA replication, but specifically blocks the serine 345 phosphorylation of the DNA damage checkpoint kinase Chk1 by Rad3 (ATR) at broken replication forks. DBAN is particularly damaging for cells with defects in the lagging-strand DNA polymerase delta. This sensitivity can be explained by the dependency of pol delta mutants on Chk1 activation for survival. We conclude that DBAN targets a process or protein that acts at the start of S phase and is required for Chk1 phosphorylation. Taken together, DBAN may precipitate cancer by perturbing S phase and by blocking the Chk1-dependent response to replication fork damage.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Cantor KP, et al. Drinking water source and chlorination byproducts in Iowa. III. Risk of brain cancer. Am. J. Epidemiol. 1999;150:552–560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

