The bulky and the sweet: How neutralizing antibodies and glycan receptors compete for virus binding
- PMID: 28986957
- PMCID: PMC5699497
- DOI: 10.1002/pro.3319
The bulky and the sweet: How neutralizing antibodies and glycan receptors compete for virus binding
Abstract
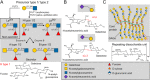
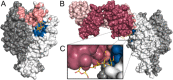
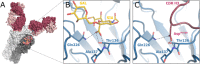
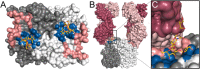
Numerous viruses rely on glycan receptor binding as the initial step in host cell infection. Engagement of specific glycan receptors such as sialylated carbohydrates, glycosaminoglycans, or histo-blood group antigens can determine host range, tissue tropism, and pathogenicity. Glycan receptor-binding sites are typically located in exposed regions on viral surfaces-sites that are also generally prone to binding of neutralizing antibodies that directly interfere with virus-glycan receptor interactions. In this review, we examine the locations and architecture of the glycan- and antibody-binding sites in four different viruses with stalk-like attachment proteins (reovirus, influenza virus, norovirus, and coronavirus) and investigate the mechanisms by which antibodies block glycan recognition. Those viruses exemplify that direct molecular mimicking of glycan receptors by antibodies is rare and further demonstrate that antibodies often partly overlap or bind sufficiently close to the receptor-binding region to hinder access to this site, achieving neutralization partially because of the epitope location and partly due to their sheer size.
Keywords: glycan receptors; neutralizing antibodies; structural characterization of binding epitopes and modes; viruses.
© 2017 The Protein Society.
Figures

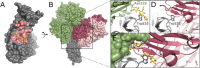

References
-
- Varki A, Schauer R (2009) Sialic acids In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, Eds. Essentials of Glycobiology, 2nd ed NY: Cold Spring Harbor. - PubMed
-
- Esko JD, Kimata K, Lindahl U (2009) Proteoglycans and sulfated glycosaminoglycans In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, Eds. Essentials of Glycobiology, 2nd ed NY: Cold Spring Harbor. - PubMed
-
- Marionneau S, Cailleau‐Thomas A, Rocher J, Le Moullac‐Vaidye B, Ruvoen N, Clement M, Le Pendu J (2001) ABH and Lewis histo‐blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 83:565–573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

