Inflammatory demyelinating diseases of the central nervous system
- PMID: 28987175
- PMCID: PMC7149979
- DOI: 10.1016/B978-0-12-802395-2.00019-5
Inflammatory demyelinating diseases of the central nervous system
Abstract
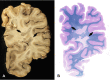
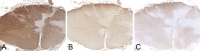
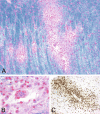
Inflammatory demyelinating diseases are a heterogeneous group of disorders, which occur against the background of an acute or chronic inflammatory process. The pathologic hallmark of multiple sclerosis (MS) is the presence of focal demyelinated lesions with partial axonal preservation and reactive astrogliosis. Demyelinated plaques are present in the white as well as gray matter, such as the cerebral or cerebellar cortex and brainstem nuclei. Activity of the disease process is reflected by the presence of lesions with ongoing myelin destruction. Axonal and neuronal destruction in the lesions is a major substrate for permanent neurologic deficit in MS patients. The MS pathology is qualitatively similar in different disease stages, such as relapsing remitting MS or secondary or primary progressive MS, but the prevalence of different lesion types differs quantitatively. Acute MS and Balo's type of concentric sclerosis appear to be variants of classic MS. In contrast, neuromyelitis optica (NMO) and spectrum disorders (NMOSD) are inflammatory diseases with primary injury of astrocytes, mediated by aquaporin-4 antibodies. Finally, we discuss the histopathology of other inflammatory demyelinating diseases such as acute disseminated encephalomyelitis and myelin oligodendrocyte glycoprotein antibody-associated demyelination. Knowledge of the heterogenous immunopathology in demyelinating diseases is important, to understand the clinical presentation and disease course and to find the optimal treatment for an individual patient.
Keywords: MOG antibodies; acute disseminated encephalomyelitis; aquaporin-4 antibodies; human autoimmune encephalomyelitis; multiple sclerosis; neuromyelitis optica.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures









References
-
- Aboul-Enein F., Rauschka H., Kornek B. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. - PubMed
-
- Aloisi F., Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nature reviews. Immunology. 2006;6:205–217. - PubMed
-
- Anlar B., Basaran C., Kose G. Acute disseminated encephalomyelitis in children: outcome and prognosis. Neuropediatrics. 2003;34:194–199. - PubMed
-
- Balo J. Encephalitis periaxialis concentrica. Arch Neurol Psychiatry. 1928;19:242–264.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

