Favorable biodistribution, specific targeting and conditional endosomal escape of RNA nanoparticles in cancer therapy
- PMID: 28987384
- PMCID: PMC5844565
- DOI: 10.1016/j.canlet.2017.09.043
Favorable biodistribution, specific targeting and conditional endosomal escape of RNA nanoparticles in cancer therapy
Abstract
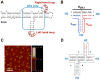
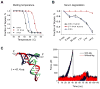
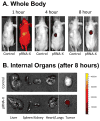
The past decades have witnessed the successful transition of several nanotechnology platforms into the clinical trials. However, specific delivery of therapeutics to tumors is hindered by several barriers including cancer recognition and tissue penetration, particle heterogeneity and aggregation, and unfavorable pharmacokinetic profiles such as fast clearance and organ accumulation. With the advent of RNA nanotechnology, a series of RNA nanoparticles have been successfully constructed to overcome many of the aforementioned challenges for in vivo cancer targeting with favorable biodistribution profiles. Compared to other nanodelivery platforms, the physiochemical properties of RNA nanoparticles can be tuned with relative ease for investigating the in vivo behavior of nanoparticles upon systemic injection. The size, shape, and surface chemistry, especially hydrophobic modifications, exert significant impacts on the in vivo fate of RNA nanoparticles. Rationally designed RNA nanoparticles with defined stoichiometry and high homogeneity have been demonstrated to specifically target tumor cells while avoiding accumulation in healthy vital organs after systemic injection. RNA nanoparticles were proven to deliver therapeutics such as siRNA and anti-miRNA to block tumor growth in several animal models. Although the release of anti-miRNA from the RNA nanoparticles has achieved high efficiency of tumor regression in multiple animal models, the efficiency of endosomal escape for siRNA delivery needs further improvement. This review focuses on the advances and perspectives of this promising RNA nanotechnology platform for cancer targeting and therapy.
Keywords: Biodistribution; Cancer therapy; Nanobiotechnology; RNA nanotechnology; pRNA-3WJ motif; phi29 motor pRNA.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures

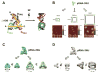


Similar articles
-
Systemic Delivery of Anti-miRNA for Suppression of Triple Negative Breast Cancer Utilizing RNA Nanotechnology.ACS Nano. 2015 Oct 27;9(10):9731-40. doi: 10.1021/acsnano.5b02471. Epub 2015 Sep 21. ACS Nano. 2015. PMID: 26387848 Free PMC article.
-
Fabrication of 14 different RNA nanoparticles for specific tumor targeting without accumulation in normal organs.RNA. 2013 Jun;19(6):767-77. doi: 10.1261/rna.037002.112. Epub 2013 Apr 19. RNA. 2013. PMID: 23604636 Free PMC article.
-
Using Planar Phi29 pRNA Three-Way Junction to Control Size and Shape of RNA Nanoparticles for Biodistribution Profiling in Mice.Methods Mol Biol. 2017;1632:359-380. doi: 10.1007/978-1-4939-7138-1_23. Methods Mol Biol. 2017. PMID: 28730451
-
Stable RNA nanoparticles as potential new generation drugs for cancer therapy.Adv Drug Deliv Rev. 2014 Feb;66:74-89. doi: 10.1016/j.addr.2013.11.006. Epub 2013 Nov 22. Adv Drug Deliv Rev. 2014. PMID: 24270010 Free PMC article. Review.
-
Delivery and biodistribution of siRNA for cancer therapy: challenges and future prospects.Ther Deliv. 2012 Feb;3(2):245-61. doi: 10.4155/tde.11.155. Ther Deliv. 2012. PMID: 22834200 Review.
Cited by
-
Nanoscale delivery systems for microRNAs in cancer therapy.Cell Mol Life Sci. 2020 Mar;77(6):1059-1086. doi: 10.1007/s00018-019-03317-9. Epub 2019 Oct 21. Cell Mol Life Sci. 2020. PMID: 31637450 Free PMC article. Review.
-
Polymer-Based Hybrid Nanoarchitectures for Cancer Therapy Applications.Polymers (Basel). 2022 Jul 26;14(15):3027. doi: 10.3390/polym14153027. Polymers (Basel). 2022. PMID: 35893988 Free PMC article. Review.
-
Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver siRNA for Cancer Suppression.Sci Rep. 2018 Oct 2;8(1):14644. doi: 10.1038/s41598-018-32953-7. Sci Rep. 2018. PMID: 30279553 Free PMC article.
-
3D RNA nanocage for encapsulation and shielding of hydrophobic biomolecules to improve the in vivo biodistribution.Nano Res. 2020 Dec;13(12):3241-3247. doi: 10.1007/s12274-020-2996-1. Epub 2020 Sep 4. Nano Res. 2020. PMID: 34484616 Free PMC article.
-
Multifunctional Nanomaterials: Recent Advancements in Cancer Therapeutics and Vaccines.Indian J Microbiol. 2025 Mar;65(1):51-68. doi: 10.1007/s12088-024-01274-x. Epub 2024 May 26. Indian J Microbiol. 2025. PMID: 40371018
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

