Organised screening for cervical cancer in France: a cost-effectiveness assessment
- PMID: 28988162
- PMCID: PMC5640017
- DOI: 10.1136/bmjopen-2016-014626
Organised screening for cervical cancer in France: a cost-effectiveness assessment
Abstract
Objective: According to the third cancer plan, organised screening (OS) of cervical cancer (CC) among women aged 25-65 years should be implemented in France in the forthcoming years. The most efficient way to implement OS in the French healthcare system is yet to be determined.
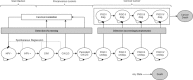
Methods: A microsimulation model was developed adopting a collective 'all payers' perspective. A closed cohort of women eligible for CC screening and representative in terms of age and participation in individual screening (IndScr) by annual Papanicolaou (Pap) testing every 3 years was modelled on a lifetime horizon. Different OS strategies, additive to IndScr with a 61.9% participation rate based on mailed invitations to non-participant women to perform OS were assessed. Similar modalities were applied to OS and IndScr participants. Strategies implied different screening tests (Papanicolaou (Pap) test, human papillomavirus (HPV) test and p16/Ki67 double staining) and OS periodicity.
Results: Compared with IndScr only, all OS strategies were associated with decreased cancer incidence/mortality (from 14.2%/13.5% to 22.9%/25.8%). Most strategies generated extra costs ranging from €37.9 to €1607 per eligible woman. HPV testing every 10 and 5 years were cost saving. HPV tests every 10 and 5 years were the most efficient strategies, generating more survival at lower costs than Pap-based strategies. Compared to IndScr only, an HPV test every 10 years was cost saving. The most effective strategies were p16/Ki67 as primary or HPV positive confirmation tests, with respective incremental cost-effectiveness ratios of €6 541 250 and €101 391 per life year. Pap-based strategies generated intermediary results.
Conclusion: OS strategies based on the HPV test appear highly efficient. However, our results rely on the assumption that women and practitioners comply with the recommended OS periodicities (3, 5, 10 years). Implementing these OS modalities will require major adaptations to the current CC screening organisation. Pap test-based strategies might be simpler to setup while preparing an appropriate implementation of more efficient OS screening modalities.
Keywords: gynaecological oncology; health economics; public health.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures



Similar articles
-
Benefits and costs of using HPV testing to screen for cervical cancer.JAMA. 2002 May 8;287(18):2372-81. doi: 10.1001/jama.287.18.2372. JAMA. 2002. PMID: 11988058
-
Cost-effectiveness of HPV-based cervical cancer screening in the public health system in Nicaragua.BMJ Open. 2017 Jun 15;7(6):e015048. doi: 10.1136/bmjopen-2016-015048. BMJ Open. 2017. PMID: 28619772 Free PMC article.
-
Reducing overtreatment associated with overdiagnosis in cervical cancer screening-A model-based benefit-harm analysis for Austria.Int J Cancer. 2020 Aug 15;147(4):1131-1142. doi: 10.1002/ijc.32849. Epub 2020 Jan 13. Int J Cancer. 2020. PMID: 31872420
-
[New strategies for the prevention and control of cervical cancer in Chile.].Salud Publica Mex. 2018 Nov-Dic;60(6):713-721. doi: 10.21149/8577. Salud Publica Mex. 2018. PMID: 30699276 Review. Spanish.
-
Human papillomavirus testing in primary cervical screening and abnormal Papanicolaou management.Obstet Gynecol Surv. 2006 Jun;61(6 Suppl 1):S15-25. doi: 10.1097/01.ogx.0000221011.01750.25. Obstet Gynecol Surv. 2006. PMID: 16729900 Review.
Cited by
-
Gender gap in annual preventive care services in France.EClinicalMedicine. 2022 May 27;49:101469. doi: 10.1016/j.eclinm.2022.101469. eCollection 2022 Jul. EClinicalMedicine. 2022. PMID: 35747180 Free PMC article.
-
A systematic review of the cost-effectiveness of interventions to increase cervical cancer screening among underserved women in Europe.Eur J Health Econ. 2024 Jul;25(5):829-844. doi: 10.1007/s10198-023-01627-1. Epub 2023 Sep 20. Eur J Health Econ. 2024. PMID: 37726429 Free PMC article.
-
Downregulation of Krüppel‑like factor 1 inhibits the metastasis and invasion of cervical cancer cells.Mol Med Rep. 2018 Oct;18(4):3932-3940. doi: 10.3892/mmr.2018.9401. Epub 2018 Aug 20. Mol Med Rep. 2018. PMID: 30132534 Free PMC article.
-
High-risk human papillomavirus cervical infection prevalence: a nationwide retrospective study comparing opportunistic and organised screening, France, 2020 to 2023.Euro Surveill. 2025 Jul;30(28):2400689. doi: 10.2807/1560-7917.ES.2025.30.28.2400689. Euro Surveill. 2025. PMID: 40682451 Free PMC article.
-
Towards equity in organised cancer screening: the case of cervical cancer screening in France.BMC Womens Health. 2018 Nov 26;18(1):192. doi: 10.1186/s12905-018-0683-0. BMC Womens Health. 2018. PMID: 30477482 Free PMC article.
References
-
- Santé HAde. État des lieux et recommandations pour le dépistage du cancer du col de l’utérus en France. 2010. http://www.has-sante.fr/portail/jcms/c_1009772/fr/etat-des-lieux-et-reco...
-
- Monnereau A, Remontet L M, et al. . Estimation nationale de l’incidence des cancers en France entre 1980 et 2012. Etude à partir des registres des cancers du réseau Francim - Partie 1 : tumeurs solides. 2013. http://invs.santepubliquefrance.fr/Publications-et-outils/Rapports-et-sy...
-
- Institut National du Cancer. Généralisation du dépistage du cancer du col de l’utérus /Étude médico-économique /Phase 1. 2015. http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publicat...
-
- Ministère des Affaires sociales et de la santé Ministère de l’Enseignement supérieur et de la recherche. Plan Cancer 2014-2019, Guérir et prévenir les cancers : donnons les mêmes chances à tous, partout en France. 2014. http://www.e-cancer.fr/Plan-cancer/Plan-cancer-2014-2019-priorites-et-ob...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
