Macrophage Extracellular Traps: A Scoping Review
- PMID: 28988241
- PMCID: PMC6757166
- DOI: 10.1159/000480373
Macrophage Extracellular Traps: A Scoping Review
Abstract
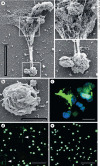
Tissue macrophages are derived from either circulating blood monocytes that originate in the bone marrow, or embryonic precursors that establish residence in tissues and are maintained independent of bone marrow progenitors. Macrophages perform diverse functions including tissue repair, the maintenance of homeostasis, and immune regulation. Recent studies have demonstrated that macrophages produce extracellular traps (ETs). ETs are an immune response by which a cell undergoes "ETosis" to release net-like material, with strands composed of cellular DNA that is studded with histones and cellular proteins. ETs are thought to immobilize and kill microorganisms, but also been implicated in disease pathology including aseptic inflammation and autoimmune disease. We conducted a scoping review to define what is known from the existing literature about the ETs produced by monocytes or macrophages. The results suggest that macrophage ETs (METs) are produced in response to various microorganisms and have similar features to neutrophil ETs (NETs), in that METs are produced by a unique cell death program (METosis), which results in release of fibers composed of DNA and studded with cellular proteins. METs function to immobilize and kill some microorganisms, but may also play a role in disease pathology.
Keywords: Extracellular traps; METosis; Macrophage.
© 2017 S. Karger AG, Basel.
Figures
References
-
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma'ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ, Immunological Genome Consortium Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. - PMC - PubMed
-
- Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. - PubMed
-
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

