Economic Burden of Herpes Zoster and Post-Herpetic Neuralgia in Adults 60 Years of Age or Older: Results from a Prospective, Physician Practice-Based Cohort Study in Kushiro, Japan
- PMID: 28988331
- PMCID: PMC5684048
- DOI: 10.1007/s40801-017-0119-4
Economic Burden of Herpes Zoster and Post-Herpetic Neuralgia in Adults 60 Years of Age or Older: Results from a Prospective, Physician Practice-Based Cohort Study in Kushiro, Japan
Abstract
Background and objective: Herpes zoster has a high incidence rate among people aged ≥ 60 years and can lead to serious complications such as post-herpetic neuralgia. There are currently no data on the economic burden of herpes zoster and post-herpetic neuralgia in Japan, and the objective of this study was to address this gap.
Methods: A total of 412 patients aged ≥ 60 years diagnosed with herpes zoster were recruited. Demographic, clinical, and healthcare resource utilization data on patients with herpes zoster or post-herpetic neuralgia collected via case report forms were used to estimate direct medical cost. Data obtained from a questionnaire survey among patients with herpes zoster/post-herpetic neuralgia were used to estimate transportation cost and productivity loss.
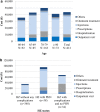
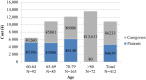
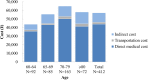
Results: The mean number of outpatient visits was 5.7. Prescription medications were the main cost driver accounting for 60% of the direct medical cost. The mean direct medical and total herpes zoster-related costs per patient were ¥43,925 and ¥57,112, respectively, and were higher in patients with post-herpetic neuralgia than in those with herpes zoster without complications. Direct medical cost represented 77%, productivity loss 19%, and transportation cost 4% of the total.
Conclusions: This is the first study of the economic burden of herpes zoster and post-herpetic neuralgia in Japan and it demonstrated substantial direct medical cost as a result of the multiple outpatient visits and prescription medications required. These findings provide baseline data for possible future economic evaluations of new herpes zoster/post-herpetic neuralgia interventions.
Trial registration: This cost analysis is part of a prospective, physician practice-based cohort study conducted between June 2013 and February 2015 in Kushiro, Japan (Clinicaltrials.gov identifier NCT01873365, registered on 6 June, 2013).
Conflict of interest statement
Funding
GlaxoSmithKline Biologicals SA funded the study and was involved in all study activities and overall data management (collection, analysis, and interpretation), and funded all costs associated with developing and publishing this article. All authors had full access to the data, and the corresponding author was responsible for submission of the publication.
Conflict of interest
K. Adachi and K. Asano reported a study grant and personal fees for medical advice from the GSK group of companies during the conduct of the study and personal fees for lectures from the GSK group of companies outside the submitted work. RA and MK reported a study grant and personal fees for medical advice from the GSK group of companies during the conduct of the study. HN reported a study grant, personal fees for medical advice, and travel support to a congress presentation from the GSK group of companies during the conduct of the study. KK reported a study grant from the GSK group of companies during the conduct of the study. AW reported a study grant from the GSK group of companies during the conduct of the study and personal fees for lectures from the GSK group of companies outside the submitted work. KH, DC, KS, and TK are employees of the GSK group of companies and hold stock options or restricted shares. TM and AM are employees of the GSK group of companies. SM is a freelance consultant working on behalf of the GSK group of companies. KS, TK, TM, and AM received funding from Japan Vaccine Co. Ltd. (a 50%/50% Joint Venture of GSK/Daiichi Sankyo Company, Ltd.) for the conduct of the study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was reviewed by the Ethics Committee of Kushiro City General Hospital, Kushiro Red Cross Hospital, or Clinical Research Hospital Tokyo.
Data availability
The cost data were collected as part of an observational, prospective, physician practice-based cohort study of people 60 years of age or older conducted in Kushiro, Japan between June 2013 and February 2015. The details of the study are published elsewhere.
Trademark
Neurotropin® is a trademark of the Nippon Zoki Pharmaceutical Co. Ltd, Japan.
Figures
Similar articles
-
Burden of herpes zoster and post-herpetic neuralgia in Sweden.BMC Infect Dis. 2015 May 22;15:215. doi: 10.1186/s12879-015-0951-7. BMC Infect Dis. 2015. PMID: 26002038 Free PMC article.
-
Acute/subacute herpes zoster: healthcare resource utilisation and costs in a group of US health plans.Pharmacoeconomics. 2007;25(2):155-69. doi: 10.2165/00019053-200725020-00007. Pharmacoeconomics. 2007. PMID: 17249857
-
Impact of Herpes Zoster and Post-Herpetic Neuralgia on Health-Related Quality of Life in Japanese Adults Aged 60 Years or Older: Results from a Prospective, Observational Cohort Study.Clin Drug Investig. 2018 Jan;38(1):29-37. doi: 10.1007/s40261-017-0581-5. Clin Drug Investig. 2018. PMID: 29086340 Free PMC article.
-
Herpes zoster--predicting and minimizing the impact of post-herpetic neuralgia.J Antimicrob Chemother. 2001 Feb;47 Suppl T1:1-8. doi: 10.1093/jac/47.suppl_1.1. J Antimicrob Chemother. 2001. PMID: 11160029 Review.
-
Epidemiology, treatment and prevention of herpes zoster: A comprehensive review.Indian J Dermatol Venereol Leprol. 2018 May-Jun;84(3):251-262. doi: 10.4103/ijdvl.IJDVL_1021_16. Indian J Dermatol Venereol Leprol. 2018. PMID: 29516900 Review.
Cited by
-
The Economic Burden and Impact on Quality of Life of Herpes Zoster and Postherpetic Neuralgia in Individuals Aged 50 Years or Older in Italy.Open Forum Infect Dis. 2019 Jan 12;6(2):ofz007. doi: 10.1093/ofid/ofz007. eCollection 2019 Feb. Open Forum Infect Dis. 2019. PMID: 30793003 Free PMC article.
-
A systematic literature review of the epidemiology and burden of herpes zoster in selected locales in Asia Pacific.Hum Vaccin Immunother. 2024 Dec 31;20(1):2344983. doi: 10.1080/21645515.2024.2344983. Epub 2024 May 20. Hum Vaccin Immunother. 2024. PMID: 38767209 Free PMC article.
-
Impact of Reactogenicity After Two Doses of Recombinant Zoster Vaccine Upon Physical Functioning and Quality of Life: An Open Phase III Trial in Older Adults.J Gerontol A Biol Sci Med Sci. 2021 Feb 25;76(3):485-490. doi: 10.1093/gerona/glaa127. J Gerontol A Biol Sci Med Sci. 2021. PMID: 32530462 Free PMC article. Clinical Trial.
-
Burden of Herpes Zoster in the Japanese Population with Immunocompromised/Chronic Disease Conditions: Results from a Cohort Study Claims Database from 2005-2014.Dermatol Ther (Heidelb). 2019 Mar;9(1):117-133. doi: 10.1007/s13555-018-0268-8. Epub 2018 Nov 19. Dermatol Ther (Heidelb). 2019. PMID: 30456446 Free PMC article.
-
The Potential Public Health Impact of Herpes Zoster Vaccination of People Aged ≥ 50 Years in Japan: Results of a Markov Model Analysis.Dermatol Ther (Heidelb). 2018 Jun;8(2):269-284. doi: 10.1007/s13555-018-0236-3. Epub 2018 Apr 21. Dermatol Ther (Heidelb). 2018. PMID: 29680914 Free PMC article.
References
-
- National Institute of Infectious Diseases. Infectious agents surveillance report (IASR). 2016. Available from: http://www.niid.go.jp/niid/ja/iasr.html. Accessed 25 Aug 2017.
-
- Centers for Disease Control and Prevention. Shingles (herpes zoster). 2016. Available from: http://www.cdc.gov/shingles/about/overview.html. Accessed 25 Aug 2017.
-
- Takao Y, Miyazaki Y, Okeda M, Onishi F, Yano S, Gomi Y, et al. Incidences of herpes zoster and postherpetic neuralgia in Japanese adults aged 50 years and older from a community-based prospective cohort study: the SHEZ Study. J Epidemiol. 2015;25:617–625. doi: 10.2188/jea.JE20140210. - DOI - PMC - PubMed
-
- Official Statistics of Japan. Total population, October 1, 2014. Available from: http://www.e-stat.go.jp. Accessed 25 Aug 2017.
-
- United Nations. Population ageing and development. 2012. Available from: http://www.un.org/esa/population/publications/2012PopAgeingDev_Chart/201.... Accessed 25 Aug 2017.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous