Phase I study of sorafenib and tipifarnib for recurrent glioblastoma: NABTC 05-02
- PMID: 28988377
- PMCID: PMC5756101
- DOI: 10.1007/s11060-017-2624-4
Phase I study of sorafenib and tipifarnib for recurrent glioblastoma: NABTC 05-02
Abstract
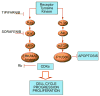
Recurrent glioblastoma (GBM) has a very low 6-month progression free survival (PFS) with currently available treatments. Combination chemotherapy to target multiple cell signaling pathways is currently being investigated in order to improve prognosis for recurrent disease. The purpose of this phase I study was to determine the maximum tolerated dose (MTD) for the combination of tipifarnib and sorafenib for the treatment of recurrent GBM. Patients with pathologically proven WHO grade IV GBM and radiographically proven tumor recurrence were eligible for this study. Treatments included sorafenib at twice daily and escalating dosages of tipifarnib. Dose-limiting toxicity (DLT) was determined over the first 28-days of treatments, and the MTD was determined in a 3 + 3 study design. We enrolled 24 patients, and 21 patients completed the MTD period. The study was stopped early with no MTD determination for excessive toxicities. The last dose level reached was sorafenib at 200 mg twice a day and tipifarnib 100 mg twice a day on an alternating week schedule. The DLTs included diarrhea, lipase elevation, hypophosphatemia, and arthralgia. The combination of sorafenib and tipifarnib has excessive toxicities and full single agent dosages could not be achieved in combination.
Keywords: Combination study; Recurrent GBM; Sorafenib; Tipifarnib.
Conflict of interest statement
Figures
Similar articles
-
Phase 1/2 trial of temsirolimus and sorafenib in the treatment of patients with recurrent glioblastoma: North Central Cancer Treatment Group Study/Alliance N0572.Cancer. 2018 Apr 1;124(7):1455-1463. doi: 10.1002/cncr.31219. Epub 2018 Jan 3. Cancer. 2018. PMID: 29313954 Free PMC article. Clinical Trial.
-
Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study.J Clin Oncol. 2006 Aug 1;24(22):3651-6. doi: 10.1200/JCO.2006.06.2323. J Clin Oncol. 2006. PMID: 16877733 Clinical Trial.
-
Phase I/II study of sorafenib in combination with temsirolimus for recurrent glioblastoma or gliosarcoma: North American Brain Tumor Consortium study 05-02.Neuro Oncol. 2012 Dec;14(12):1511-8. doi: 10.1093/neuonc/nos264. Epub 2012 Oct 24. Neuro Oncol. 2012. PMID: 23099651 Free PMC article. Clinical Trial.
-
Phase I trial of tipifarnib (R115777) concurrent with radiotherapy in patients with glioblastoma multiforme.Int J Radiat Oncol Biol Phys. 2007 Aug 1;68(5):1396-401. doi: 10.1016/j.ijrobp.2007.02.043. Epub 2007 Jun 14. Int J Radiat Oncol Biol Phys. 2007. PMID: 17570606 Clinical Trial.
-
Vandetanib plus sirolimus in adults with recurrent glioblastoma: results of a phase I and dose expansion cohort study.J Neurooncol. 2015 Feb;121(3):627-34. doi: 10.1007/s11060-014-1680-2. Epub 2014 Dec 13. J Neurooncol. 2015. PMID: 25503302 Free PMC article. Clinical Trial.
Cited by
-
Glioblastoma Stem Cells-Useful Tools in the Battle against Cancer.Int J Mol Sci. 2022 Apr 21;23(9):4602. doi: 10.3390/ijms23094602. Int J Mol Sci. 2022. PMID: 35562993 Free PMC article. Review.
-
Advanced tumor electric fields therapy: A review of innovative research and development and prospect of application in glioblastoma.CNS Neurosci Ther. 2024 May;30(5):e14720. doi: 10.1111/cns.14720. CNS Neurosci Ther. 2024. PMID: 38715344 Free PMC article. Review.
-
Tenascin-cmediated vasculogenic mimicry formation via regulation of MMP2/MMP9 in glioma.Cell Death Dis. 2019 Nov 21;10(12):879. doi: 10.1038/s41419-019-2102-3. Cell Death Dis. 2019. PMID: 31754182 Free PMC article.
-
Cell Membrane-Coated Magnetic Nanocubes with a Homotypic Targeting Ability Increase Intracellular Temperature due to ROS Scavenging and Act as a Versatile Theranostic System for Glioblastoma Multiforme.Adv Healthc Mater. 2019 Sep;8(18):e1900612. doi: 10.1002/adhm.201900612. Epub 2019 Aug 7. Adv Healthc Mater. 2019. PMID: 31389193 Free PMC article.
-
PALB2 deficiency may sensitize H3K27M-mutant pediatric HGG cells to BMN673/talazoparib.Front Oncol. 2025 Jun 30;15:1589396. doi: 10.3389/fonc.2025.1589396. eCollection 2025. Front Oncol. 2025. PMID: 40661778 Free PMC article.
References
-
- American Cancer Society. Cancer Facts & Figures 2010. American Cancer Society; Atlanta: 2010.
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO the European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups and the National Cancer Institute of Canada Clinical Trials G. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. New England Journal of Medicine. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. - DOI - PubMed
-
- Wong E, Hess K, Gleason M, Jaeckle K, APK, MDP, VAL, WKY Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. Journal of Clinical Oncology. 1999;17(8):2572. - PubMed
-
- Lamborn KR, Yung WKA, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM, Robins HI, Lieberman FS, Fine HA, Fink KL, Junck L, Abrey L, Gilbert MR, Mehta M, Kuhn JG, Aldape KD, Hibberts J, Peterson PM, Prados MD North American Brain Tumor C. Progression-free survival: An important end point in evaluating therapy for recurrent high-grade gliomas. Neuro-Oncology. 2008;10(2):162–170. doi: 10.1215/15228517-2007-062. - DOI - PMC - PubMed
-
- Friedman HS, Prados M, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. Journal of Clinical Oncology. 2009;27(28):4733–4740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical