Control of HIV infection by IFN-α: implications for latency and a cure
- PMID: 28988399
- PMCID: PMC11105398
- DOI: 10.1007/s00018-017-2652-4
Control of HIV infection by IFN-α: implications for latency and a cure
Abstract
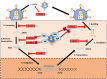
Viral infections, including HIV, trigger the production of type I interferons (IFNs), which in turn, activate a signalling cascade that ultimately culminates with the expression of anti-viral proteins. Mounting evidence suggests that type I IFNs, in particular IFN-α, play a pivotal role in limiting acute HIV infection. Highly active anti-retroviral treatment reduces viral load and increases life expectancy in HIV positive patients; however, it fails to fully eliminate latent HIV reservoirs. To revisit HIV as a curable disease, this article reviews a body of literature that highlights type I IFNs as mediators in the control of HIV infection, with particular focus on the anti-HIV restriction factors induced and/or activated by IFN-α. In addition, we discuss the relevance of type I IFN treatment in the context of HIV latency reversal, novel therapeutic intervention strategies and the potential for full HIV clearance.
Keywords: Anti-viral; Cure; HIV; Interferon; JAK/STAT; Latency.
Figures

References
-
- Hertzog PJ, Bourke NM, de Weerd NA, Mangan NE. New interferons A2. In: Ratcliffe MJH, editor. Encyclopedia of immunobiology. Oxford: Academic Press; 2016. pp. 501–508.
-
- Hardy GA, Sieg S, Rodriguez B, Anthony D, Asaad R, Jiang W, Mudd J, Schacker T, Funderburg NT, Pilch-Cooper HA, et al. Interferon-α is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS One. 2013;8:e56527. doi: 10.1371/journal.pone.0056527. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

