Antimicrobial Peptide Combined with BMP2-Modified Mesenchymal Stem Cells Promotes Calvarial Repair in an Osteolytic Model
- PMID: 28988712
- PMCID: PMC5762982
- DOI: 10.1016/j.ymthe.2017.09.011
Antimicrobial Peptide Combined with BMP2-Modified Mesenchymal Stem Cells Promotes Calvarial Repair in an Osteolytic Model
Abstract
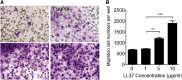
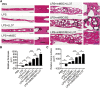
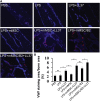
Repair and regeneration of inflammation-induced bone loss remains a clinical challenge. LL37, an antimicrobial peptide, plays critical roles in cell migration, cytokine production, apoptosis, and angiogenesis. Migration of stem cells to the affected site and promotion of vascularization are essential for tissue engineering therapy, including bone regeneration. However, it is largely unknown whether LL37 affects mesenchymal stem cell (MSC) behavior and bone morphogenetic protein 2 (BMP2)-mediated bone repair during the bone pathologic remodeling process. By performing in vitro and in vivo studies with MSCs and a lipopolysaccharide (LPS)-induced mouse calvarial osteolytic bone defect model, we found that LL37 significantly promotes cell differentiation, migration, and proliferation in both unmodified MSCs and BMP2 gene-modified MSCs. Additionally, LL37 inhibited LPS-induced osteoclast formation and bacterial activity in vitro. Furthermore, the combination of LL37 and BMP2 markedly promoted MSC-mediated angiogenesis and bone repair and regeneration in LPS-induced osteolytic defects in mouse calvaria. These findings demonstrate for the first time that LL37 can be a potential candidate drug for promoting osteogenesis and for inhibiting bacterial growth and osteoclastogenesis, and that the combination of BMP2 and LL37 is ideal for MSC-mediated bone regeneration, especially for inflammation-induced bone loss.
Keywords: LL37; antimicrobial peptide; bone loss; bone regeneration; bone repair; inflammation; mesenchymal stem cells; osteolysis.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Overview of signal transduction between LL37 and bone marrow-derived MSCs.J Mol Histol. 2022 Apr;53(2):149-157. doi: 10.1007/s10735-021-10048-4. Epub 2022 Jan 20. J Mol Histol. 2022. PMID: 35048213 Review.
-
The combination of nano-calcium sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified mesenchymal stem cells promotes bone regeneration in rat critical-sized calvarial defects.Stem Cell Res Ther. 2017 May 25;8(1):122. doi: 10.1186/s13287-017-0574-6. Stem Cell Res Ther. 2017. PMID: 28545565 Free PMC article.
-
The antimicrobial peptide LL37 promotes bone regeneration in a rat calvarial bone defect.Peptides. 2013 Aug;46:136-42. doi: 10.1016/j.peptides.2013.06.001. Epub 2013 Jun 12. Peptides. 2013. PMID: 23770151
-
Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect.Acta Biomater. 2018 Sep 15;78:36-47. doi: 10.1016/j.actbio.2018.07.050. Epub 2018 Jul 29. Acta Biomater. 2018. PMID: 30067947
-
Genetically Engineered-MSC Therapies for Non-unions, Delayed Unions and Critical-size Bone Defects.Int J Mol Sci. 2019 Jul 12;20(14):3430. doi: 10.3390/ijms20143430. Int J Mol Sci. 2019. PMID: 31336890 Free PMC article. Review.
Cited by
-
Peptide-Based Biomaterials for Bone and Cartilage Regeneration.Biomedicines. 2024 Jan 29;12(2):313. doi: 10.3390/biomedicines12020313. Biomedicines. 2024. PMID: 38397915 Free PMC article. Review.
-
Cathelicidin LL-37 in Health and Diseases of the Oral Cavity.Biomedicines. 2022 May 7;10(5):1086. doi: 10.3390/biomedicines10051086. Biomedicines. 2022. PMID: 35625823 Free PMC article. Review.
-
Biomaterials and nanomedicine for bone regeneration: Progress and future prospects.Exploration (Beijing). 2021 Oct 30;1(2):20210011. doi: 10.1002/EXP.20210011. eCollection 2021 Oct. Exploration (Beijing). 2021. PMID: 37323213 Free PMC article.
-
Self-assembly of PEG-PPS polymers and LL-37 peptide nanomicelles improves the oxidative microenvironment and promotes angiogenesis to facilitate chronic wound healing.Bioeng Transl Med. 2023 Nov 8;9(2):e10619. doi: 10.1002/btm2.10619. eCollection 2024 Mar. Bioeng Transl Med. 2023. PMID: 38435813 Free PMC article.
-
Overview of signal transduction between LL37 and bone marrow-derived MSCs.J Mol Histol. 2022 Apr;53(2):149-157. doi: 10.1007/s10735-021-10048-4. Epub 2022 Jan 20. J Mol Histol. 2022. PMID: 35048213 Review.
References
-
- Hardy R., Cooper M.S. Bone loss in inflammatory disorders. J. Endocrinol. 2009;201:309–320. - PubMed
-
- Souza P.P., Lerner U.H. The role of cytokines in inflammatory bone loss. Immunol. Invest. 2013;42:555–622. - PubMed
-
- Redlich K., Smolen J.S. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012;11:234–250. - PubMed
-
- Trombone A.P., Ferreira S.B., Jr., Raimundo F.M., de Moura K.C., Avila-Campos M.J., Silva J.S., Campanelli A.P., De Franco M., Garlet G.P. Experimental periodontitis in mice selected for maximal or minimal inflammatory reactions: increased inflammatory immune responsiveness drives increased alveolar bone loss without enhancing the control of periodontal infection. J. Periodontal Res. 2009;44:443–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

