IRX3 Promotes the Browning of White Adipocytes and Its Rare Variants are Associated with Human Obesity Risk
- PMID: 28988979
- PMCID: PMC5652024
- DOI: 10.1016/j.ebiom.2017.09.010
IRX3 Promotes the Browning of White Adipocytes and Its Rare Variants are Associated with Human Obesity Risk
Abstract
Background: IRX3 was recently reported as the effector of the FTO variants. We aimed to test IRX3's roles in the browning program and to evaluate the association between the genetic variants in IRX3 and human obesity.
Methods: IRX3 expression was examined in beige adipocytes in human and mouse models, and further validated in induced beige adipocytes. The browning capacity of primary preadipocytes was assessed with IRX3 knockdown. Luciferase reporter analysis and ChIP assay were applied to investigate IRX3's effects on UCP1 transcriptional activity. Moreover, genetic analysis of IRX3 was performed in 861 young obese subjects and 916 controls.
Results: IRX3 expression was induced in the browning process and was positively correlated with the browning markers. IRX3 knockdown remarkably inhibited UCP1 expression in induced mouse and human beige adipocytes, and also repressed the uncoupled oxygen consumption rate. Further, IRX3 directly bound to UCP1 promoter and increased its transcriptional activity. Moreover, 17 rare heterozygous missense/frameshift IRX3 variants were identified, with a significant enrichment in obese subjects (P=0.038, OR=2.27; 95% CI, 1.02-5.05).
Conclusions: IRX3 deficiency repressed the browning program of white adipocytes partially by regulating UCP1 transcriptional activity. Rare variants of IRX3 were associated with human obesity.
Keywords: Beige adipocytes; Browning program; Genetic variants; IRX3; Obesity.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
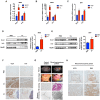

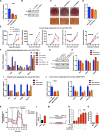
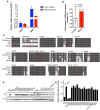
Comment in
-
Regulations of Adipocyte Phenotype and Obesity by IRX3. Positive or Negative?EBioMedicine. 2017 Oct;24:7-8. doi: 10.1016/j.ebiom.2017.09.032. Epub 2017 Sep 25. EBioMedicine. 2017. PMID: 28967609 Free PMC article. No abstract available.
Similar articles
-
IRX3 Overexpression Enhances Ucp1 Expression In Vivo.Front Endocrinol (Lausanne). 2021 Mar 10;12:634191. doi: 10.3389/fendo.2021.634191. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33776928 Free PMC article.
-
Irisin exerts dual effects on browning and adipogenesis of human white adipocytes.Am J Physiol Endocrinol Metab. 2016 Aug 1;311(2):E530-41. doi: 10.1152/ajpendo.00094.2016. Epub 2016 Jul 19. Am J Physiol Endocrinol Metab. 2016. PMID: 27436609
-
FTO Obesity Risk Variants Are Linked to Adipocyte IRX3 Expression and BMI of Children - Relevance of FTO Variants to Defend Body Weight in Lean Children?PLoS One. 2016 Aug 25;11(8):e0161739. doi: 10.1371/journal.pone.0161739. eCollection 2016. PLoS One. 2016. PMID: 27560134 Free PMC article.
-
Meaningful respirometric measurements of UCP1-mediated thermogenesis.Biochimie. 2017 Mar;134:56-61. doi: 10.1016/j.biochi.2016.12.005. Epub 2016 Dec 14. Biochimie. 2017. PMID: 27986537 Review.
-
Understanding the Biology of Thermogenic Fat: Is Browning A New Approach to the Treatment of Obesity?Arch Med Res. 2017 Jul;48(5):401-413. doi: 10.1016/j.arcmed.2017.10.002. Arch Med Res. 2017. PMID: 29102386 Review.
Cited by
-
IRX3 Overexpression Enhances Ucp1 Expression In Vivo.Front Endocrinol (Lausanne). 2021 Mar 10;12:634191. doi: 10.3389/fendo.2021.634191. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33776928 Free PMC article.
-
FTO haplotyping underlines high obesity risk for European populations.BMC Med Genomics. 2019 Mar 13;12(Suppl 2):46. doi: 10.1186/s12920-019-0491-x. BMC Med Genomics. 2019. PMID: 30871540 Free PMC article.
-
Family-Based Quantitative Trait Meta-Analysis Implicates Rare Noncoding Variants in DENND1A in Polycystic Ovary Syndrome.J Clin Endocrinol Metab. 2019 Sep 1;104(9):3835-3850. doi: 10.1210/jc.2018-02496. J Clin Endocrinol Metab. 2019. PMID: 31038695 Free PMC article.
-
Common genetic variation in obesity, lipid transfer genes and risk of Metabolic Syndrome: Results from IDEFICS/I.Family study and meta-analysis.Sci Rep. 2020 Apr 28;10(1):7189. doi: 10.1038/s41598-020-64031-2. Sci Rep. 2020. PMID: 32346024 Free PMC article.
-
Dynamic chromatin architecture of the porcine adipose tissues with weight gain and loss.Nat Commun. 2023 Jun 12;14(1):3457. doi: 10.1038/s41467-023-39191-0. Nat Commun. 2023. PMID: 37308492 Free PMC article.
References
-
- Allison D.B., Kaprio J., Korkeila M., Koskenvuo M., Neale M.C., Hayakawa K. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int. J. Obes. Relat. Metab. Disord. 1996;20:501–506. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

