Diversity-oriented synthesis of heterocycles and macrocycles by controlled reactions of oxetanes with α-iminocarbenes
- PMID: 28989611
- PMCID: PMC5621157
- DOI: 10.1039/c7sc00964j
Diversity-oriented synthesis of heterocycles and macrocycles by controlled reactions of oxetanes with α-iminocarbenes
Abstract
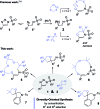
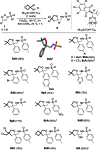
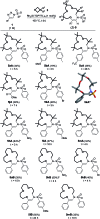
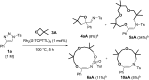
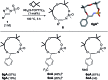
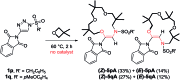
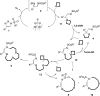
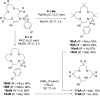
Using N-sulfonyl triazoles as substrates, compounds as diverse as 2-imino tetrahydrofurans, 13- and 15-membered ring aza-macrocycles can be prepared selectively via formal [1 + 4], [5 + 4 + 4] and [3 + 4 + 4 + 4] condensations of α-imino carbenes and oxetanes under Rh(ii)-catalysis or thermal activation. Spirocyclic N-heterocycles are also accessible by means of Buchwald-Hartwig and Pictet-Spengler cyclizations. By reaction control, substrate selection or further derivatization, a large variety of chemical structures is thus achievable. Finally, using triazoles reacting under thermal activation, interesting mechanistic insight was obtained.
Figures


References
-
- Kolb H. C., Finn M. G., Sharpless K. B. Angew. Chem., Int. Ed. 2001;40:2004–2021. - PubMed
- Lewis W. G., Green L. G., Grynszpan F., Radić Z., Carlier P. R., Taylor P., Finn M. G., Sharpless K. B. Angew. Chem., Int. Ed. 2002;41:1053–1057. - PubMed
- Amblard F., Cho J. H., Schinazi R. F. Chem. Rev. 2009;109:4207–4220. - PMC - PubMed
- Le Droumaguet C., Wang C., Wang Q. Chem. Soc. Rev. 2010;39:1233–1239. - PubMed
- Thirumurugan P., Matosiuk D., Jozwiak K. Chem. Rev. 2013;113:4905–4979. - PubMed
-
- Harmon R. E., Stanley F., Gupta S. K., Johnson J. J. Org. Chem. 1970;35:3444–3448.
- Harmon R. E., Earl R. A., Gupta S. K. J. Chem. Soc. D. 1971:296–297.
-
- Chattopadhyay B., Gevorgyan V. Angew. Chem., Int. Ed. 2012;51:862–872. - PMC - PubMed
- Gulevich A. V., Gevorgyan V. Angew. Chem., Int. Ed. 2013;52:1371–1373. - PMC - PubMed
- Davies H. M. L., Alford J. S. Chem. Soc. Rev. 2014;43:5151–5162. - PubMed
- Anbarasan P., Yadagiri D., Rajasekar S. Synthesis. 2014;46:3004–3023.
- Wang Y., Lei X., Tang Y. Synlett. 2015;26:2051–2059.
- Jiang Y., Sun R., Tang X.-Y., Shi M. Chem. – Eur. J. 2016;22:17910–17924. - PubMed
-
-
For selected Rh-catalyzed reactions of triazoles, see the following articles on C–H, C–O, C–S, N–H or O–H bond insertions:
- Chuprakov S., Malik J. A., Zibinsky M., Fokin V. V. J. Am. Chem. Soc. 2011;133:10352–10355. - PMC - PubMed
- Yadagiri D., Anbarasan P. Chem. – Eur. J. 2013;19:15115–15119. - PubMed
- Fu J., Shen H., Chang Y., Li C., Gong J., Yang Z. Chem. – Eur. J. 2014;20:12881–12888. - PubMed
- Miura T., Tanaka T., Matsumoto K., Murakami M. Chem. – Eur. J. 2014;20:16078–16082. - PubMed
- Jeon H. J., Jung D. J., Kim J. H., Kim Y., Bouffard J., Lee S.-g. J. Org. Chem. 2014;79:9865–9871. - PubMed
- Miura T., Fujimoto Y., Funakoshi Y., Murakami M. Angew. Chem., Int. Ed. 2015;54:9967–9970. - PubMed
- Lindsay V. N. G., Viart H. M. F., Sarpong R. J. Am. Chem. Soc. 2015;137:8368–8371. - PubMed
- Shen M.-H., Pan Y.-P., Jia Z.-H., Ren X.-T., Zhang P., Xu H.-D. Org. Biomol. Chem. 2015;13:4851–4854. - PubMed
- Li L., Xia X.-H., Wang Y., Bora P. P., Kang Q. Adv. Synth. Catal. 2015;357:2089–2097.
- Ma X., Wu F., Yi X., Wang H., Chen W. Chem. Commun. 2015;51:6862–6865. - PubMed
- Lee D. J., Yoo E. J. Org. Lett. 2015;17:1830–1833. - PubMed
- Shi Y., Yu X., Li C.-Y. Eur. J. Org. Chem. 2015;2015:6429–6433.
- Senoo M., Furukawa A., Hata T., Urabe H. Chem. – Eur. J. 2016;22:890–895. - PubMed
- Kubiak R. W., Mighion J. D., Wilkerson-Hill S. M., Alford J. S., Yoshidomi T., Davies H. M. L. Org. Lett. 2016;18:3118–3121. - PMC - PubMed
- Miura T., Nakamuro T., Miyakawa S., Murakami M. Angew. Chem., Int. Ed. 2016;55:8732–8735. - PubMed
- Chuprakov S., Kwok S. W., Zhang L., Lercher L., Fokin V. V. J. Am. Chem. Soc. 2009;131:18034–18035. - PMC - PubMed
- Zibinsky M., Fokin V. V. Org. Lett. 2011;13:4870–4872. - PMC - PubMed
- Miura T., Nakamuro T., Liang C.-J., Murakami M. J. Am. Chem. Soc. 2014;136:15905–15908. - PubMed
- Rajagopal B., Chou C.-H., Chung C.-C., Lin P.-C. Org. Lett. 2014;16:3752–3755. - PubMed
- Yadagiri D., Anbarasan P. Org. Lett. 2014;16:2510–2513. - PubMed
- Shin S., Park Y., Kim C.-E., Son J.-Y., Lee P. H. J. Org. Chem. 2015;80:5859–5869. - PubMed
- Seo B., Jeon W. H., Kim J., Kim S., Lee P. H. J. Org. Chem. 2015;80:722–732. - PubMed
- Tang X.-Y., Zhang Y.-S., He L., Wei Y., Shi M. Chem. Commun. 2015;51:133–136. - PubMed
- Meng J., Ding X., Yu X., Deng W.-P. Tetrahedron. 2016;72:176–183.
- Chuprakov S., Hwang F. W., Gevorgyan V. Angew. Chem., Int. Ed. 2007;46:4757–4759. - PMC - PubMed
- Schultz E. E., Sarpong R. J. Am. Chem. Soc. 2013;135:4696–4699. - PubMed
- Spangler J. E., Davies H. M. L. J. Am. Chem. Soc. 2013;135:6802–6805. - PubMed
- Shen H., Fu J., Gong J., Yang Z. Org. Lett. 2014;16:5588–5591. - PubMed
- Jung D. J., Jeon H. J., Lee J. H., Lee S.-g. Org. Lett. 2015;17:3498–3501. - PubMed
- Ding H., Hong S., Zhang N. Tetrahedron Lett. 2015;56:507–510.
- Lee E., Ryu T., Shin E., Son J.-Y., Choi W., Lee P. H. Org. Lett. 2015;17:2470–2473. - PubMed
- Miura T., Funakoshi Y., Fujimoto Y., Nakahashi J., Murakami M. Org. Lett. 2015;17:2454–2457. - PubMed
- He J., Man Z., Shi Y., Li C.-Y. J. Org. Chem. 2015;80:4816–4823. - PubMed
- Zhang W.-B., Xiu S.-D., Li C.-Y. Org. Chem. Front. 2015;2:47–50.
- Lei X., Li L., He Y.-P., Tang Y. Org. Lett. 2015;17:5224–5227. - PubMed
- Kim S., Kim J. E., Lee J., Lee P. H. Adv. Synth. Catal. 2015;357:3707–3717.
- He J., Shi Y., Cheng W., Man Z., Yang D., Li C.-Y. Angew. Chem., Int. Ed. 2016;55:4557–4561. - PubMed
- Yadagiri D., Reddy A. C. S., Anbarasan P. Chem. Sci. 2016;7:5934. - PMC - PubMed
- Chen W., Bai Y.-L., Luo Y.-C., Xu P.-F. Org. Lett. 2017;19:364–367. - PubMed
- Jung D. J., Jeon H. J., Kim J. H., Kim Y., Lee S.-g. Org. Lett. 2014;16:2208–2211. - PubMed
- Xing Y., Sheng G., Wang J., Lu P., Wang Y. Org. Lett. 2014;16:1244–1247. - PubMed
- Ran R.-Q., Xiu S.-D., Li C.-Y. Org. Lett. 2014;16:6394–6396. - PubMed
- Zhang Y.-S., Tang X.-Y., Shi M. Chem. Commun. 2014;50:15971–15974. - PubMed
- Yang Y., Zhou M.-B., Ouyang X.-H., Pi R., Song R.-J., Li J.-H. Angew. Chem., Int. Ed. 2015;54:6595–6599. - PubMed
- Kim C.-E., Park Y., Park S., Lee P. H. Adv. Synth. Catal. 2015;357:210–220.
- Lee D. J., Ko D., Yoo E. J. Angew. Chem., Int. Ed. 2015;54:13715–13718. - PubMed
- Wang H., Qiao H., Zhang H., Yang H., Zhao Y., Fu H. Eur. J. Org. Chem. 2015:4471–4480.
- Jiang Y., Tang X.-Y., Shi M. Chem. Commun. 2015;51:2122–2125. - PubMed
- Tian Y., Wang Y., Shang H., Xu X., Tang Y. Org. Biomol. Chem. 2015;13:612–619. - PubMed
- Zhang H., Wang H., Yang H., Fu H. Org. Biomol. Chem. 2015;13:6149–6153. - PubMed
- Xu H.-D., Xu K., Zhou H., Jia Z.-H., Wu H., Lu X.-L., Shen M.-H. Synthesis. 2015;47:641–646.
- Xu H.-D., Jia Z.-H., Xu K., Zhou H., Shen M.-H. Org. Lett. 2015;17:66–69. - PubMed
- Sun R., Jiang Y., Tang X.-Y., Shi M. Chem. – Eur. J. 2016;22:5727–5733. - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources

