Stimuli-responsive nucleic acid-functionalized metal-organic framework nanoparticles using pH- and metal-ion-dependent DNAzymes as locks
- PMID: 28989617
- PMCID: PMC5621505
- DOI: 10.1039/c7sc01765k
Stimuli-responsive nucleic acid-functionalized metal-organic framework nanoparticles using pH- and metal-ion-dependent DNAzymes as locks
Abstract
A versatile approach to modify metal-organic framework nanoparticles (NMOFs) with nucleic acid tethers, using the "click chemistry" method is introduced. The nucleic acid-functionalized NMOFs are used to prepare stimuli-responsive carriers of loads (fluorescence probes or anti-cancer drugs). Two different stimuli-responsive nucleic acid-based NMOFs are presented. One system involves the preparation of pH-responsive NMOFs. The NMOFs are loaded with fluorophores or doxorubicin anti-cancer drug and locked in the NMOFs by pH-responsive DNA duplex capping units. At pH = 5.0 the capping units are unlocked, leading to the release of the loads. The AS1411 aptamer is conjugated to the locking units as the targeting unit for the nucleolin biomarker present in cancer cells. The pH-responsive doxorubicin-loaded NMOFs and, in particular, the AS1411 aptamer-modified pH-responsive NMOFs reveal selective, targeted, cytotoxicity toward MDA-MB-231 breast cancer cells. A second system involves the synthesis of NMOFs that are loaded with fluorophores or doxorubicin and capped with metal-ion-dependent DNAzyme/substrate complexes as locking units (metal ion = Mg2+ or Pb2+ ions). In the presence of the respective metal ions, the nucleic acid locking units are cleaved off, resulting in the release of the loads. Also, "smart" Mg2+-ion-dependent DNAzyme capped doxorubicin-loaded NMOFs are synthesized via the integration of the ATP aptamer sequence in the loop domain of the Mg2+-dependent DNAzyme. The unlocking of these NMOFs proceeds effectively only in the presence of ATP and Mg2+ ions, acting as cooperative triggers. As ATP is over-expressed in cancer cells, the "smart" carrier provides sense-and-treat functions. The "smart" ATP/Mg2+-triggered doxorubicin-loaded NMOFs reveal selective cytotoxicity toward MDA-MB-231 cancer cells. Beyond the use of the metal-ion-dependent DNAzymes as ion-responsive locks of drug-loaded NMOF carriers, the DNAzyme-capped fluorophore-loaded NMOFs are successfully applied as functional units for multiplexed ion-sensing and for the design of logic-gate systems.
Figures
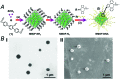






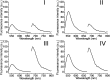



References
-
- Wang Z., Cohen S. M. Chem. Soc. Rev. 2009;38:1315–1329. - PubMed
- Furukawa H., Cordova K. E., O’Keeffe M., Yaghi O. M. Science. 2013;341:1230444. - PubMed
- Cui Y. J., Li B., He H. J., Zhou W., Chen B. L., Qian G. D. Acc. Chem. Res. 2016;49:483–493. - PubMed
- Kreno L. E., Leong K., Farha O. K., Allendorf M., Van Duyne R. P., Hupp J. T. Chem. Rev. 2012;112:1105–1125. - PubMed
-
- Yang S. J., Kim T., Im J. H., Kim Y. S., Lee K., Jung H., Park C. R. Chem. Mater. 2012;24:464–470.
- Li Y., Yang R. T. Langmuir. 2007;23:12937–12944. - PubMed
-
- He C., Lu K., Liu D., Lin W. J. Am. Chem. Soc. 2014;136:5181–5184. - PMC - PubMed
- Horcajada P., Chalati T., Serre C., Gillet B., Sebrie C., Baati T., Eubank J. F., Heurtaux D., Clayette P., Kreuz C., Chang J. S., Hwang Y. K., Marsaud V., Bories P. N., Cynober L., Gil S., Ferey G., Couvreur P., Gref R. Nat. Mater. 2010;9:172–178. - PubMed
- Liu D., Huxford R. C., Lin W. Angew. Chem., Int. Ed. 2011;50:3696–3700. - PMC - PubMed
- Horcajada P., Gref R., Baati T., Allan P. K., Maurin G., Couvreur P., Ferey G., Morris R. E., Serre C. Chem. Rev. 2012;112:1232–1268. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

