On the incompatibility of lithium-O2 battery technology with CO2
- PMID: 28989641
- PMCID: PMC5625616
- DOI: 10.1039/c7sc01230f
On the incompatibility of lithium-O2 battery technology with CO2
Abstract
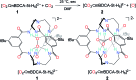
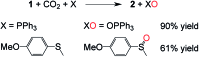
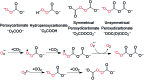
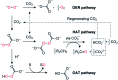
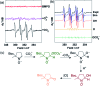
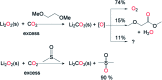
When solubilized in a hexacarboxamide cryptand anion receptor, the peroxide dianion reacts rapidly with CO2 in polar aprotic organic media to produce hydroperoxycarbonate (HOOCO2-) and peroxydicarbonate (-O2COOCO2-). Peroxydicarbonate is subject to thermal fragmentation into two equivalents of the highly reactive carbonate radical anion, which promotes hydrogen atom abstraction reactions responsible for the oxidative degradation of organic solvents. The activation and conversion of the peroxide dianion by CO2 is general. Exposure of solid lithium peroxide (Li2O2) to CO2 in polar aprotic organic media results in aggressive oxidation. These findings indicate that CO2 must not be introduced in conditions relevant to typical lithium-O2 cell configurations, as production of HOOCO2- and -O2COOCO2- during lithium-O2 cell cycling will lead to cell degradation via oxidation of organic electrolytes and other vulnerable cell components.
Figures

References
-
- Bruce P. G., Freunberger S. A., Hardwick L. J., Tarascon J.-M. Nat. Mater. 2011;11:172. - PubMed
-
- Gowda S. R., Brunet A., Wallraff G. M., McCloskey B. D. J. Phys. Chem. Lett. 2013;4:276–279. - PubMed
-
- Lim H.-K., Lim H.-D., Park K.-Y., Seo D.-H., Gwon H., Hong J., Goddard W. A., Kim H., Kang K. J. Am. Chem. Soc. 2013;135:9733–9742. - PubMed
-
- Freunberger S. A., Chen Y., Peng Z., Griffin J. M., Hardwick L. J., Bardé F., Novák P., Bruce P. G. J. Am. Chem. Soc. 2011;133:8040–8047. - PubMed
-
- Chen Y., Freunberger S. A., Peng Z., Bardé F., Bruce P. G. J. Am. Chem. Soc. 2012;134:7952–7957. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

