Trends, Costs, and Complications of Anterior Cervical Discectomy and Fusion With and Without Bone Morphogenetic Protein in the United States Medicare Population
- PMID: 28989837
- PMCID: PMC5624371
- DOI: 10.1177/2192568217699207
Trends, Costs, and Complications of Anterior Cervical Discectomy and Fusion With and Without Bone Morphogenetic Protein in the United States Medicare Population
Abstract
Study design: Retrospective database review.
Objectives: After the Food and Drug Administration approved bone morphogenetic protein-2 (BMP) in 2002, BMP was used off-label in the cervical spine to increase bone growth and bony fusion. Since then, concerns have been raised regarding complication rates and safety. This study was conducted to examine the use of BMP in anterior cervical discectomy and fusion (ACDF) in the Medicare population and to determine risk of complications and associated costs within 90 days of surgery.
Methods: Patients who underwent ACDF were identified using Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision Procedure codes (ICD9-P). Complications were identified using ICD9 diagnostic codes. Charges were calculated as amount billed, and reimbursements were calculated as amounts paid by Medicare. Data for these analyses came from a nationwide claims database.
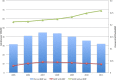
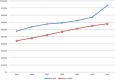
Results: A total of 215 047 patients were identified who had ACDF from 2005 to 2011. For the majority of the procedures (89.0%), BMP was not used. BMP use rose from 11.84% in 2005 to a peak of 16.73% in 2007 before decreasing to 12.01% in 2011. BMP was used 16% more in women than men. BMP use was the highest in the West (13.6%) followed by Midwest (11.8%), South (10.6%), and Northeast (7.5%). There was a higher overall complication rate in the BMP group (2.1%) compared with the non-BMP group (1.9%) (odds ratio [OR] = 1.11, 95% CI = 1.01-1.22). The BMP group also had a higher rate of wound complications (0.98% vs 0.76%, OR = 1.29, 95% CI = 1.12-1.48). In this study population, there was no difference in dysphagia/hoarseness, neurologic, medical, or other complications. During the 90-day perioperative period, BMP surgeries were charged at 17.6% higher than non-BMP surgeries.
Conclusions: The use of BMP in ACDF in the Medicare population has decreased since a peak in 2007. The rate of wound and overall complications for BMP use with ACDF was higher than without. Our results regarding dysphagia/hoarseness did not show a statistically meaningful difference, which is in contrast with many other studies. Charges associated with BMP use were higher during the 90-day perioperative period.
Keywords: anterior cervical discectomy and fusion; bone morphogenetic protein; trends.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The financial activities of the authors are as follows: S. Tim Yoon—Bioment, Stryker, Nuvasive, Medyssey, Meditech, Phygen, Alphatec, Medtronic; Jim A. Yousseff—Nuvasive, Integra, Amedica, HealthTrust, Osprey Biomedical, Vertiflex, Benvenue, Paradigm Spine, Promethean Surgical, ISD, Spinicity, Spinal Ventures, Providence Medical, Globus Medical; Darrel S. Brodke—Amedica, Depuy Synthes, Medtronic; Jeffrey C. Wang—Fziomed, Alphatech, Promethean Spine, Paradigm Spine, Benevenue, NexGen, Amedica, Vertiflex, Electrocore, Surgitech, VG Innovations, Corespine, Expanding Orthopaedics, Osprey, Bone Biologics, Curative Biosciences, Pearldiver, Stryker, Osprey, Aesculap, Biomet, Amedica, Seaspine, Synthes, North American Spine Society, North American Spine Foundation, Cervical Spine Research Society, AO Spine/AO Foundation, Collaborative Spine Research Foundation.
Figures
References
-
- Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. - PubMed
-
- Wang MC, Chan L, Maiman DJ, Kreuter W, Deyo RA. Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. Spine (Phila Pa 1976). 2007;32:342–347. - PubMed
-
- US Food and Drug Administration. InFUSE Bone Graft/LT-CAGE lumbar tapered fusion device. 2009. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApp.... Accessed 2016.
-
- Singh K, Nandyala SV, Marquez-Lara A, Fineberg SJ. Epidemiological trends in the utilization of bone morphogenetic protein in spinal fusions from 2002 to 2011. Spine (Phila Pa 1976). 2014;39:491–496. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources