Ultrasound‑targeted microbubbles combined with a peptide nucleic acid binding nuclear localization signal mediate transfection of exogenous genes by improving cytoplasmic and nuclear import
- PMID: 28990051
- PMCID: PMC5779960
- DOI: 10.3892/mmr.2017.7681
Ultrasound‑targeted microbubbles combined with a peptide nucleic acid binding nuclear localization signal mediate transfection of exogenous genes by improving cytoplasmic and nuclear import
Abstract
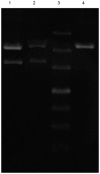
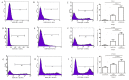
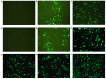
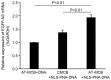
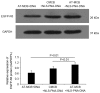
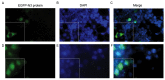
The development of an efficient delivery system is critical for the successful treatment of cardiovascular diseases using non‑viral gene therapies. Cytoplasmic and nuclear membrane barriers reduce delivery efficiency by impeding the transfection of foreign genes. Thus, a gene delivery system capable of transporting exogenous genes may improve gene therapy. The present study used a novel strategy involving ultrasound‑targeted microbubbles and peptide nucleic acid (PNA)‑binding nuclear localization signals (NLS). Ultrasound‑targeted microbubble destruction (UTMD) and PNA‑binding NLS were used to improve the cytoplasmic and nuclear importation of the plasmid, respectively. Experiments were performed using antibody‑targeted microbubbles (AT‑MCB) that specifically recognize the SV40T antigen receptor expressed on the membranes of 293T cells, resulting in the localization of ultrasound microbubbles to 293T cell membranes. Furthermore, PNA containing NLS was inserted into the enhanced green fluorescent protein (EGFP)‑N3 plasmid DNA (NLS‑PNA‑DNA), which increased nuclear localization. The nuclear import and gene expression efficiency of the AT‑MCB with PNA‑binding NLS were compared with AT‑MCB alone or a PNA‑binding NLS. The effect of the AT‑MCB containing PNA‑binding NLS on transfection was investigated. The ultrasound and AT‑MCB delivery significantly enhanced the cytoplasmic intake of exogenous genes and maintained high cell viability. The nuclear import and gene expression of combined microbubble‑ and PNA‑transfected cells were significantly greater compared with cells that were transfected with AT‑MCB or DNA with only PNA‑binding NLS. The quantity of EGFP‑N3 plasmids in the nuclei was increased by >5.0‑fold compared with control microbubbles (CMCB) and NLS‑free plasmids. The gene expression was ~1.7‑fold greater compared with NLS‑free plasmids and 1.3‑fold greater compared with control microbubbles. In conclusion, UTMD combined with AT‑MCB and a PNA‑binding NLS plasmid significantly improved transfection efficiency by increasing cytoplasmic and nuclear DNA import. This method is a promising strategy for the noninvasive and effective delivery of target genes or drugs for the treatment of cardiovascular diseases.
Figures
Similar articles
-
Enhanced effect of nuclear localization signal peptide during ultrasound‑targeted microbubble destruction‑mediated gene transfection.Mol Med Rep. 2017 Jul;16(1):565-572. doi: 10.3892/mmr.2017.6661. Epub 2017 May 31. Mol Med Rep. 2017. PMID: 28586046 Free PMC article.
-
Efficient gene therapy with a combination of ultrasound‑targeted microbubble destruction and PEI/DNA/NLS complexes.Mol Med Rep. 2017 Nov;16(5):7685-7691. doi: 10.3892/mmr.2017.7510. Epub 2017 Sep 18. Mol Med Rep. 2017. PMID: 28944824
-
Ultrasound microbubbles combined with the NFκB binding motif increase transfection efficiency by enhancing the cytoplasmic and nuclear import of plasmid DNA.Mol Med Rep. 2013 Nov;8(5):1439-45. doi: 10.3892/mmr.2013.1672. Epub 2013 Sep 9. Mol Med Rep. 2013. PMID: 24026477
-
Utilization of synthetic peptides containing nuclear localization signals for nonviral gene transfer systems.Gene Ther. 2002 Feb;9(3):157-67. doi: 10.1038/sj.gt.3301635. Gene Ther. 2002. PMID: 11859418 Review.
-
Factors influencing the nuclear targeting ability of nuclear localization signals.J Drug Target. 2016 Dec;24(10):927-933. doi: 10.1080/1061186X.2016.1184273. Epub 2016 May 24. J Drug Target. 2016. PMID: 27126810 Review.
Cited by
-
Co-assembled Ca2+ Alginate-Sulfate Nanoparticles for Intracellular Plasmid DNA Delivery.Mol Ther Nucleic Acids. 2019 Jun 7;16:378-390. doi: 10.1016/j.omtn.2019.03.006. Epub 2019 Mar 28. Mol Ther Nucleic Acids. 2019. PMID: 31003172 Free PMC article.
-
"PFH/AGM-CBA/HSV-TK/LIPOSOME-Affibody": Novel Targeted Nano Ultrasound Contrast Agents for Ultrasound Imaging and Inhibited the Growth of ErbB2-Overexpressing Gastric Cancer Cells.Drug Des Devel Ther. 2022 May 18;16:1515-1530. doi: 10.2147/DDDT.S351623. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 35611358 Free PMC article.
References
-
- Bekeredjian R, Chen S, Frenkel PA, Grayburn PA, Shohet RV. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108:1022–1026. doi: 10.1161/01.CIR.0000084535.35435.AE. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

