Metformin accelerates wound healing in type 2 diabetic db/db mice
- PMID: 28990070
- PMCID: PMC5779947
- DOI: 10.3892/mmr.2017.7707
Metformin accelerates wound healing in type 2 diabetic db/db mice
Abstract
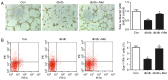
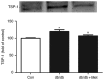
Wound healing impairment is increasingly recognized to be a consequence of hyperglycemia‑induced dysfunction of endothelial precursor cells (EPCs) in type 2 diabetes mellitus (T2DM). Metformin exhibits potential for the improvement of endothelial function and the wound healing process. However, the underlying mechanisms for the observed beneficial effects of metformin application remain to be completely understood. The present study assessed whether metformin, a widely used therapeutic drug for T2DM, may accelerate wound closure in T2DM db/db mice. Genetically hyperglycemic db/db mice were used as the T2DM model. Metformin (250 mg/kg/day; intragastric) was administered for two weeks prior to EPC collection and wound model creation in db/db mice. Wound healing was evaluated by alterations in the wound area and the number of platelet endothelial cell adhesion molecule‑positive cells. The function of the isolated bone marrow‑derived EPCs (BM‑EPCs) was assessed by a tube formation assay. The number of circulating EPCs, and the levels of intracellular nitric oxide (NO) and superoxide (O2‑) were detected by flow cytometry. Thrombospondin‑1 (TSP‑1) expression was determined by western blot analysis. It was observed that treatment with metformin accelerated wound healing, improved angiogenesis and increased the circulating EPC number in db/db mice. In vitro, treatment with metformin reversed the impaired BM‑EPC function reflected by tube formation, and significantly increased NO production while decreasing O2‑ levels in BM‑EPCs from db/db mice. In addition, TSP‑1 expression was markedly attenuated by treatment with metformin in cultured BM‑EPCs. Metformin contributed to wound healing and improved angiogenesis in T2DM mice, which was, in part, associated with stimulation of NO, and inhibition of O2‑ and TSP‑1 in EPCs from db/db mice.
Figures

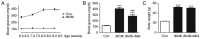



Similar articles
-
Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice.Cardiovasc Diabetol. 2016 Jun 18;15:88. doi: 10.1186/s12933-016-0408-3. Cardiovasc Diabetol. 2016. PMID: 27316923 Free PMC article.
-
Depletion of NAD pool contributes to impairment of endothelial progenitor cell mobilization in diabetes.Metabolism. 2016 Jun;65(6):852-62. doi: 10.1016/j.metabol.2016.03.006. Epub 2016 Mar 15. Metabolism. 2016. PMID: 27173464
-
Acarbose Accelerates Wound Healing via Akt/eNOS Signaling in db/db Mice.Oxid Med Cell Longev. 2017;2017:7809581. doi: 10.1155/2017/7809581. Epub 2017 Mar 8. Oxid Med Cell Longev. 2017. PMID: 28373902 Free PMC article.
-
Regulation of endothelial progenitor cell functions during hyperglycemia: new therapeutic targets in diabetic wound healing.J Mol Med (Berl). 2022 Apr;100(4):485-498. doi: 10.1007/s00109-021-02172-1. Epub 2022 Jan 8. J Mol Med (Berl). 2022. PMID: 34997250 Review.
-
Systematic review and meta-analysis of mouse models of diabetes-associated ulcers.BMJ Open Diabetes Res Care. 2020 May;8(1):e000982. doi: 10.1136/bmjdrc-2019-000982. BMJ Open Diabetes Res Care. 2020. PMID: 32467222 Free PMC article.
Cited by
-
Advances in Anti-metabolic Disease Treatments Targeting CD47.Curr Pharm Des. 2022;28(46):3720-3728. doi: 10.2174/1381612828666221006123144. Curr Pharm Des. 2022. PMID: 36201266 Review.
-
An Insight into Aging, Senescence, and Their Impacts on Wound Healing.Adv Geriatr Med Res. 2021;3(3):e210017. doi: 10.20900/agmr20210017. Epub 2021 Jul 21. Adv Geriatr Med Res. 2021. PMID: 34414398 Free PMC article.
-
Co-Delivery of Valsartan and Metformin from a Thermosensitive Hydrogel-Nanoparticle System Promotes Collagen Production in Proliferating and Senescent Primary Human Dermal Fibroblasts.Biomacromolecules. 2024 Sep 9;25(9):5702-5717. doi: 10.1021/acs.biomac.3c01461. Epub 2024 Aug 26. Biomacromolecules. 2024. PMID: 39186039 Free PMC article.
-
Metformin induces the M2 macrophage polarization to accelerate the wound healing via regulating AMPK/mTOR/NLRP3 inflammasome singling pathway.Am J Transl Res. 2019 Feb 15;11(2):655-668. eCollection 2019. Am J Transl Res. 2019. PMID: 30899369 Free PMC article.
-
Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer.Asian J Pharm Sci. 2019 Mar;14(2):130-143. doi: 10.1016/j.ajps.2018.04.004. Epub 2018 May 10. Asian J Pharm Sci. 2019. PMID: 32104445 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

