Ulinastatin inhibits renal tubular epithelial apoptosis and interstitial fibrosis in rats with unilateral ureteral obstruction
- PMID: 28990075
- PMCID: PMC5779974
- DOI: 10.3892/mmr.2017.7692
Ulinastatin inhibits renal tubular epithelial apoptosis and interstitial fibrosis in rats with unilateral ureteral obstruction
Abstract
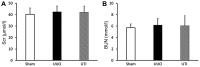
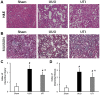
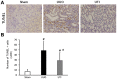
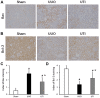
The effect of ulinastatin (UTI) on renal tubular epithelial apoptosis and interstitial fibrosis in rats with unilateral ureteral obstruction (UUO) was investigated. A total of 18 male Wistar rats were randomly divided into the following 3 groups: The Sham group (n=6), the UUO group (n=6), and the UTI group (n=6). In the UUO and UTI groups, the left ureter was ligated to establish a UUO model. Starting from day 1 after surgery, an intervention treatment was performed using normal saline (1 ml/kg/d) and UTI (40,000 unit/kg/d). On day 7 after surgery, 6 rats from each group were sacrificed. In the Sham group, the left ureter was only freed, not ligated; after 7 days of abdominal closure, all of the rats were sacrificed. Blood samples were collected prior to sacrificing the animals to measure the blood urea nitrogen (BUN) and serum creatinine (Scr). The incidence of renal interstitial lesions on the obstruction side was observed by hematoxylin and eosin, and Masson staining. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, and immunohistochemical detection of apoptosis regulator Bax (Bax), apoptosis regulator Bcl‑2 (Bcl‑2) and caspase‑3 were performed to observe the presence of renal tubular epithelial cell apoptosis. The UTI did not have a significant influence on the mouse BUN and Scr levels in any of the groups (P>0.05). Compared with that in the Sham group, renal tissue injury in the UUO group was significantly aggravated with renal tubular dilation, epithelial cell atrophy, renal interstitial inflammatory cell infiltration and fibrous tissue hyperplasia (P<0.01). Furthermore, the renal tubular epithelial TUNEL+ cell number and Bax and caspase‑3 levels were increased, and the expression of Bcl‑2 was decreased (P<0.01). Following the UTI treatment, the renal interstitial injury at the obstruction side was significantly attenuated (P<0.05), the renal tubular epithelial TUNEL+ cell number, and Bax and caspase‑3 levels significantly decreased, and the expression of Bcl‑2 was restored (P<0.05). UTI inhibited renal tubular epithelial apoptosis and interstitial fibrosis in UUO rats.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

