The effect of anagliptin on intimal hyperplasia of rat carotid artery after balloon injury
- PMID: 28990108
- PMCID: PMC5779884
- DOI: 10.3892/mmr.2017.7667
The effect of anagliptin on intimal hyperplasia of rat carotid artery after balloon injury
Abstract
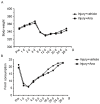
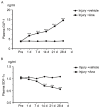
The present study evaluated the effect of anagliptin on intimal hyperplasia following carotid artery injury in Sprague‑Dawley rats. Sprague‑Dawley rats weighing 280‑300 g were injured using a 2F Fogarty balloon embolectomy catheter. The rats were divided into injury‑(saline) and anagliptin‑(10 mg/kg/day) treated groups. vascular injuries were induced in the left carotid artery, followed by evaluation of neointima formation at 28 days. The right and left carotid arteries were harvested and evaluated with histological evaluation, and the plasma activity of glucagon‑like peptide 1 receptor (GLP‑1), stromal cell‑derived factor (SDF)‑1α, interleukin (IL)‑6, IL‑1β and tumor necrosis factor (TNF)‑α were detected by ELISA analysis. Treatment with anagliptin decreased balloon injury‑induced neointima formation, compared with the injury group (P<0.01). Body weight and food consumption did not alter following treatment with anagliptin. Anagliptin caused an increase in the serum active GLP‑1 concentration, compared with the injury group. In addition, serum SDF‑1α was significantly decreased by treatment with anagliptin (P<0.001). Anagliptin altered the serum activity of IL‑6, IL‑1β and TNF‑α (P<0.01). The results of the present study demonstrated that anagliptin appeared to attenuate neointimal formation by inhibiting inflammatory cytokines and chemokines following balloon injury, and that treatment with a dipeptidyl peptidase 4 inhibitor may be useful for future preclinical studies and potentially for the inhibition of thrombosis formation following percutaneous coronary intervention.
Figures



Similar articles
-
Human urine kininogenase attenuates balloon-induced intimal hyperplasia in rabbit carotid artery through transforming growth factor β1/Smad2/3 signaling pathway.J Vasc Surg. 2016 Oct;64(4):1074-83. doi: 10.1016/j.jvs.2015.04.433. Epub 2015 Jun 6. J Vasc Surg. 2016. PMID: 26054589
-
Proresolving Lipid Mediators Resolvin D1 and Protectin D1 Isomer Attenuate Neointimal Hyperplasia in the Rat Carotid Artery Balloon Injury Model.J Surg Res. 2019 Jan;233:104-110. doi: 10.1016/j.jss.2018.07.049. Epub 2018 Aug 18. J Surg Res. 2019. PMID: 30502235
-
Hydrogen-rich saline prevents neointima formation after carotid balloon injury by suppressing ROS and the TNF-α/NF-κB pathway.Atherosclerosis. 2012 Feb;220(2):343-50. doi: 10.1016/j.atherosclerosis.2011.11.002. Epub 2011 Nov 11. Atherosclerosis. 2012. PMID: 22153150
-
Mechanisms of intimal hyperplasia learned from a murine carotid artery ligation model.Curr Vasc Pharmacol. 2008 Jan;6(1):37-43. doi: 10.2174/157016108783331321. Curr Vasc Pharmacol. 2008. PMID: 18220938 Review.
-
Inflammation as a key event in the development of neointima following vascular balloon injury.Clin Exp Pharmacol Physiol. 2001 Nov;28(11):891-5. doi: 10.1046/j.1440-1681.2001.03543.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11703391 Review.
Cited by
-
Molecular Action of Hydroxytyrosol in Attenuation of Intimal Hyperplasia: A Scoping Review.Front Pharmacol. 2021 May 21;12:663266. doi: 10.3389/fphar.2021.663266. eCollection 2021. Front Pharmacol. 2021. PMID: 34093194 Free PMC article.
-
Linagliptin protects rat carotid artery from balloon injury and activates the NRF2 antioxidant pathway.Exp Anim. 2019 Feb 26;68(1):81-90. doi: 10.1538/expanim.18-0089. Epub 2018 Oct 23. Exp Anim. 2019. PMID: 30369549 Free PMC article.
-
Platelet-Derived Microvesicles Promote VSMC Dedifferentiation After Intimal Injury via Src/Lamtor1/mTORC1 Signaling.Front Cell Dev Biol. 2021 Sep 16;9:744320. doi: 10.3389/fcell.2021.744320. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34604241 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

