Dual Src and EGFR inhibition in combination with gemcitabine in advanced pancreatic cancer: phase I results : A phase I clinical trial
- PMID: 28990119
- PMCID: PMC5891394
- DOI: 10.1007/s10637-017-0519-z
Dual Src and EGFR inhibition in combination with gemcitabine in advanced pancreatic cancer: phase I results : A phase I clinical trial
Abstract
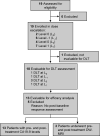
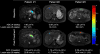
Pancreatic adenocarcinoma remains a major therapeutic challenge, as the poor (<8%) 5-year survival rate has not improved over the last three decades. Our previous preclinical data showed cooperative attenuation of pancreatic tumor growth when dasatinib (Src inhibitor) was added to erlotinib (EGFR inhibitor) and gemcitabine. Thus, this study was designed to determine the maximum-tolerated dose of the triplet combination. Standard 3 + 3 dose escalation was used, starting with daily oral doses of 70 mg dasatinib and 100 mg erlotinib with gemcitabine on days 1, 8, and 15 (800 mg/m2) of a 28-day cycle (L0). Nineteen patients were enrolled, yet 18 evaluable for dose-limiting toxicities (DLTs). One DLT observed at L0, however dasatinib was reduced to 50 mg (L-1) given side effects observed in the first two patients. At L-1, a DLT occurred in 1/6 patients and dose was re-escalated to L0, where zero DLTs reported in next four patients. Dasatinib was escalated to 100 mg (L1) where 1/6 patients experienced a DLT. Although L1 was tolerable, dose escalation was stopped as investigators felt L1 was within the optimal therapeutic window. Most frequent toxicities were anemia (89%), elevated aspartate aminotransferase (79%), fatigue (79%), nausea (79%), elevated alanine aminotransferase (74%), lymphopenia (74%), leukopenia (74%), neutropenia (63%), and thrombocytopenia (63%), most Grade 1/2. Stable disease as best response was observed in 69% (9/13). Median progression-free and overall survival was 3.6 and 8 months, respectively. Dasatinib, erlotinib, and gemcitabine was safe with manageable side effects, and with encouraging preliminary clinical activity in advanced pancreatic cancer.
Keywords: Dasatinib; Dual Src and EGFR inhibition; Erlotinib; Pancreatic cancer.
Figures

Similar articles
-
Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth.Clin Cancer Res. 2011 Feb 1;17(3):483-93. doi: 10.1158/1078-0432.CCR-10-1670. Epub 2011 Jan 25. Clin Cancer Res. 2011. PMID: 21266529 Free PMC article.
-
A phase I study of gemcitabine + dasatinib (gd) or gemcitabine + dasatinib + cetuximab (GDC) in refractory solid tumors.Cancer Chemother Pharmacol. 2019 Jun;83(6):1025-1035. doi: 10.1007/s00280-019-03805-6. Epub 2019 Mar 20. Cancer Chemother Pharmacol. 2019. PMID: 30895346 Free PMC article. Clinical Trial.
-
Erlotinib is effective in pancreatic cancer with epidermal growth factor receptor mutations: a randomized, open-label, prospective trial.Oncotarget. 2015 Jul 20;6(20):18162-73. doi: 10.18632/oncotarget.4216. Oncotarget. 2015. PMID: 26046796 Free PMC article. Clinical Trial.
-
A phase 1/1B trial of ADI-PEG 20 plus nab-paclitaxel and gemcitabine in patients with advanced pancreatic adenocarcinoma.Cancer. 2017 Dec 1;123(23):4556-4565. doi: 10.1002/cncr.30897. Epub 2017 Aug 18. Cancer. 2017. PMID: 28832976 Clinical Trial.
-
A phase I study of erlotinib in combination with gemcitabine and radiation in locally advanced, non-operable pancreatic adenocarcinoma.Ann Oncol. 2008 Jan;19(1):86-91. doi: 10.1093/annonc/mdm441. Epub 2007 Sep 17. Ann Oncol. 2008. PMID: 17878176 Clinical Trial.
Cited by
-
HER2 Overexpression in Periampullary Tumors According to Anatomical and Histological Classification-A Systematic Review.J Pers Med. 2024 Apr 27;14(5):463. doi: 10.3390/jpm14050463. J Pers Med. 2024. PMID: 38793045 Free PMC article. Review.
-
The State-of-the-Art of Phase II/III Clinical Trials for Targeted Pancreatic Cancer Therapies.J Clin Med. 2021 Feb 3;10(4):566. doi: 10.3390/jcm10040566. J Clin Med. 2021. PMID: 33546207 Free PMC article. Review.
-
Dasatinib can enhance paclitaxel and gemcitabine inhibitory activity in human pancreatic cancer cells.Cancer Biol Ther. 2019;20(6):855-865. doi: 10.1080/15384047.2019.1579956. Epub 2019 Mar 13. Cancer Biol Ther. 2019. PMID: 30866697 Free PMC article.
-
Exploration of the System-Level Mechanisms of the Herbal Drug FDY003 for Pancreatic Cancer Treatment: A Network Pharmacological Investigation.Evid Based Complement Alternat Med. 2022 May 10;2022:7160209. doi: 10.1155/2022/7160209. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35591866 Free PMC article.
-
Combined Src/EGFR Inhibition Targets STAT3 Signaling and Induces Stromal Remodeling to Improve Survival in Pancreatic Cancer.Mol Cancer Res. 2020 Apr;18(4):623-631. doi: 10.1158/1541-7786.MCR-19-0741. Epub 2020 Jan 16. Mol Cancer Res. 2020. PMID: 31949002 Free PMC article.
References
-
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Groupe Tumeurs Digestives of U, Intergroup P FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. - DOI - PubMed
-
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. - DOI - PMC - PubMed
-
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National Cancer Institute of Canada Clinical Trials G Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

