Diagnostic Accuracy of FebriDx: A Rapid Test to Detect Immune Responses to Viral and Bacterial Upper Respiratory Infections
- PMID: 28991170
- PMCID: PMC5664009
- DOI: 10.3390/jcm6100094
Diagnostic Accuracy of FebriDx: A Rapid Test to Detect Immune Responses to Viral and Bacterial Upper Respiratory Infections
Abstract
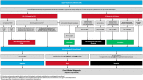
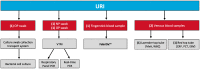
C-reactive protein (CRP) and myxovirus resistance protein A (MxA) are associated with bacterial and viral infections, respectively. We conducted a prospective, multicenter, cross-sectional study of adults and children with febrile upper respiratory tract infections (URIs) to evaluate the diagnostic accuracy of a rapid CRP/MxA immunoassay to identify clinically significant bacterial infection with host response and acute pathogenic viral infection. The reference standard for classifying URI etiology was an algorithm that included throat bacterial culture, upper respiratory PCR for viral and atypical pathogens, procalcitonin, white blood cell count, and bandemia. The algorithm also allowed for physician override. Among 205 patients, 25 (12.2%) were classified as bacterial, 53 (25.9%) as viral, and 127 (62.0%) negative by the reference standard. For bacterial detection, agreement between FebriDx and the reference standard was 91.7%, with FebriDx having a sensitivity of 80% (95% CI: 59-93%), specificity of 93% (89-97%), positive predictive value (PPV) of 63% (45-79%), and a negative predictive value (NPV) of 97% (94-99%). For viral detection, agreement was 84%, with a sensitivity of 87% (75-95%), specificity of 83% (76-89%), PPV of 64% (63-75%), and NPV of 95% (90-98%). FebriDx may help to identify clinically significant immune responses associated with bacterial and viral URIs that are more likely to require clinical management or therapeutic intervention, and has potential to assist with antibiotic stewardship.
Keywords: CRP; FebriDx; MxA; antibiotic stewardship; immunoassay; respiratory infection.
Conflict of interest statement
This work was supported by RPS Diagnostics, the maker of FebriDx. The authors received research funding from RPS Diagnostics to conduct this study. The sponsor participated in the design of the study. The authors independently collected, analyzed, and interpreted the data; prepared the manuscript, and made the decision to publish the results. Self reports serving as a paid consultant for BioFire Diagnostics/BioMerieux and Ferring Pharmaceuticals, and receiving research funding from BioMerieux and ThermoFisher/BRAHMS. Hou has served as a paid consultant for Cheetah Medical. Kurz has received honoraria from Zoll Medical Corporation, and has received research funding from Zoll Medical Corporation and Boehringer-Ingelheim. Shapiro has served as a paid consultant for Cheetah Medical, and has received research funding from Thermo Fisher, Siemens, and Cumberland pharma.
Figures
References
-
- Dellit T.H., Owens R.C., McGowan J.E., Jr., Gerding D.N., Weinstein R.A., Burke J.P., Huskins W.C., Paterson D.L., Fishman N.O., Carpenter C.F., et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007;44:159–177. doi: 10.1086/510393. - DOI - PubMed
-
- Centers for Disease Control and Prevention Get Smart: Know when Antibiotics Work. [(accessed on 5 May 2016)]; Available online: http://www.cdc.gov/getsmart/community/index.html.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous