Furanones and Anthranilic Acid Derivatives from the Endophytic Fungus Dendrothyrium variisporum
- PMID: 28991218
- PMCID: PMC6151570
- DOI: 10.3390/molecules22101674
Furanones and Anthranilic Acid Derivatives from the Endophytic Fungus Dendrothyrium variisporum
Abstract
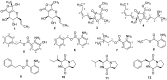
Extracts from an endophytic fungus isolated from the roots of the Algerian plant Globularia alypum showed prominent antimicrobial activity in a screening for novel antibiotics. The producer organism was identified as Dendrothyrium variisporum by means of morphological studies and molecular phylogenetic methods. Studies on the secondary metabolite production of this strain in various culture media revealed that the major components from shake flasks were massarilactones D (1) and H (2) as well as two new furanone derivatives for which we propose the trivial names (5S)-cis-gregatin B (3) and graminin D (4). Scale-up of the fermentation in a 10 L bioreactor yielded massarilactone D and several further metabolites. Among those were three new anthranilic acid derivatives (5-7), two known anthranilic acid analogues (8 and 9) and three cyclopeptides (10-12). Their structures were elucidated on the basis of extensive spectroscopic analysis (1D- and 2D-NMR), high-resolution mass spectrometry (HRESIMS), and the application of the modified Mosher's method. The isolated metabolites were tested for antimicrobial and cytotoxic activities against various bacteria, fungi, and two mammalian cell lines. The new Metabolite 5 and Compound 9 exhibited antimicrobial activity while Compound 9 showed cytotoxicity activity against KB3.1 cells.
Keywords: Dendrothyrium variisporum; Montagnulaceae; anthranilic acid derivatives; antimicrobial activity; cytotoxicity; fermentation; furancarboxylic acid derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Cytotoxic anthranilic acid derivatives from deep sea sediment-derived fungus Penicillium paneum SD-44.Mar Drugs. 2013 Aug 21;11(8):3068-76. doi: 10.3390/md11083068. Mar Drugs. 2013. PMID: 23966037 Free PMC article.
-
Antimicrobial properties of unusual eremophilanes from the endophytic Diaporthe sp. BCC69512.Phytochemistry. 2024 Jun;222:114078. doi: 10.1016/j.phytochem.2024.114078. Epub 2024 Apr 3. Phytochemistry. 2024. PMID: 38574958
-
Pestalafuranones F-J, five new furanone analogues from the endophytic fungus Nigrospora sp. BM-2.Molecules. 2014 Jan 10;19(1):819-25. doi: 10.3390/molecules19010819. Molecules. 2014. PMID: 24434694 Free PMC article.
-
Furanones.Prog Mol Subcell Biol. 2006;42:55-86. doi: 10.1007/3-540-30016-3_2. Prog Mol Subcell Biol. 2006. PMID: 16805438 Review.
-
Medicinal chemistry of anthranilic acid derivatives: A mini review.Drug Dev Res. 2021 Nov;82(7):945-958. doi: 10.1002/ddr.21842. Epub 2021 Jun 12. Drug Dev Res. 2021. PMID: 34117784 Review.
Cited by
-
Biological Activities of Some New Secondary Metabolites Isolated from Endophytic Fungi: A Review Study.Int J Mol Sci. 2021 Jan 19;22(2):959. doi: 10.3390/ijms22020959. Int J Mol Sci. 2021. PMID: 33478038 Free PMC article. Review.
-
Fungal Endophytes: A Potential Source of Antibacterial Compounds.J Fungi (Basel). 2022 Feb 8;8(2):164. doi: 10.3390/jof8020164. J Fungi (Basel). 2022. PMID: 35205918 Free PMC article. Review.
-
Lasiodipline G and other diketopiperazine metabolites produced by Lasiodiplodia chiangraiensis.RSC Adv. 2023 Jun 27;13(28):19373-19378. doi: 10.1039/d3ra03242f. eCollection 2023 Jun 22. RSC Adv. 2023. PMID: 37383691 Free PMC article.
-
In Vitro Effects of Fungal Phytotoxins on Cancer Cell Viability: First Insight into Structure Activity Relationship of a Potent Metabolite of Cochliobolus australiensis Radicinin.Toxins (Basel). 2022 Jul 29;14(8):517. doi: 10.3390/toxins14080517. Toxins (Basel). 2022. PMID: 36006179 Free PMC article.
-
Synthesis, Molecular Docking, and Biological Evaluation of Novel Anthranilic Acid Hybrid and Its Diamides as Antispasmodics.Int J Mol Sci. 2023 Sep 8;24(18):13855. doi: 10.3390/ijms241813855. Int J Mol Sci. 2023. PMID: 37762158 Free PMC article.
References
-
- Karwehl S., Stadler M. Exploitation of fungal biodiversity for discovery of novel antibiotics. Curr. Top. Microbiol. Immunol. 2016;398:303–338. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

