Alu RNA accumulation induces epithelial-to-mesenchymal transition by modulating miR-566 and is associated with cancer progression
- PMID: 28991230
- PMCID: PMC5799714
- DOI: 10.1038/onc.2017.369
Alu RNA accumulation induces epithelial-to-mesenchymal transition by modulating miR-566 and is associated with cancer progression
Abstract
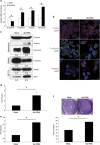
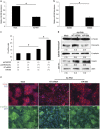
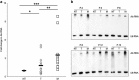
Alu sequences are the most abundant short interspersed repeated elements in the human genome. Here we show that in a cell culture model of colorectal cancer (CRC) progression, we observe accumulation of Alu RNA that is associated with reduced DICER1 levels. Alu RNA induces epithelial-to-mesenchymal transition (EMT) by acting as a molecular sponge of miR-566. Moreover, Alu RNA accumulates as consequence of DICER1 deficit in colorectal, ovarian, renal and breast cancer cell lines. Interestingly, Alu RNA knockdown prevents DICER1 depletion-induced EMT despite global microRNA (miRNA) downregulation. Alu RNA expression is also induced by transforming growth factor-β1, a major driver of EMT. Corroborating this data, we found that non-coding Alu RNA significantly correlates with tumor progression in human CRC patients. Together, these findings reveal an unexpected DICER1-dependent, miRNA-independent role of Alu RNA in cancer progression that could bring mobile element transcripts in the fields of cancer therapeutic and prognosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wang W, Wang WH, Azadzoi KM, Dai P, Wang Q, Sun JB et al. Alu RNA accumulation in hyperglycemia augments oxidative stress and impairs eNOS and SOD2 expression in endothelial cells. Mol Cell Endocrinol 2016; 426: 91–100. - PubMed
-
- Panning B, Smiley JR. Activation of RNA polymerase III transcription of human Alu elements by herpes simplex virus. Virology 1994; 202: 408–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

